This judgment was handed down remotely at 12 noon on Thursday 8th May 2025 by circulation to the parties or their representatives by e-mail and by release to the National Archives.
.............................
Lord Justice Green:
A. Introduction
The applications
1. The issues before the Court concern the legality of various models of pricing for the sale of generic pharmaceutical drugs to the NHS . The Competition and Markets Authority (“CMA”) issued a decision, running to nearly 650 pages, in which it found that a supplier had abused a dominant position by increasing the price of a generic, long out of patent, drug designed to treat thyroid deficiency to excessive and legally unfair levels in sales to the NHS. Between 2009 and 2017 the price rose from £20 per box to £247 per box. The CMA held that there was no objective justification for these price increases and imposed penalties exceeding £100m. Following an appeal on the merits of that decision to the Competition Appeal Tribunal (“CAT”) a judgment was handed down running to 498 paragraphs dismissing the appeals, save in relation to one narrow point on penalties.
2. The applications before the Court are for permission to appeal from the judgment of the CAT with the appeals to follow immediately if permission is granted. The judgment (“the Judgment”) is dated 8th August 2023 ([2023] CAT 52). This upheld the decision of the CMA dated 29th July 2021 (“the Decision”). This found that, contrary to section 18 Competition Act 1998 (“CA 1998”), a single undertaking, identified as “Advanz”, abused a dominant position from 1st January 2009 to 1st July 2017 (the “Infringement Period”). The Decision is entitled “Excessive and unfair pricing with respect to the supply of liothyronine tablets in the UK”.
The applicants
3. The applications are from the Cinven and Advanz Pharma Corp groups of companies - individually “Cinven” and “Advanz Pharma Corp” and collectively “the applicants” and/or “Advanz”. In the Decision, Advanz was treated as a single “undertaking”. However, at various points in time over the Infringement Period the entities which made up Advanz changed. The CAT (Judgment paragraph [1]) described these changes in the following way:
“…Between 2007 and 2017, a single undertaking consisting of Mercury Pharmaceuticals Limited, Advanz Pharma Services (UK) Limited and Mercury Pharma Group Limited (“the Mercury Pharma Companies”) and, at various points, the Hg Appellant, the Cinven Appellants and Advanz Pharma Corp Limited (“Advanz Pharma Corp”), was the sole supplier of 20mcg liothyronine sodium tablets (“Liothyronine Tablets”) in the UK. This single undertaking as it existed at any particular point is referred to in this Judgment as “Advanz”.”
The penalties
4. The CMA concluded that Advanz made an unlawful profit of over £92.3 million from the infringement, of which:
- £5.7 million dated from the period when Advanz was controlled by HgCapital (“Hg”);
- £34.1 million dated from the period when Advanz was controlled by the Cinven applicants; and
- £52.5 million dated from the period when Advanz was controlled by Advanz Pharma Corp.
5. The CMA imposed a total financial penalty of £101,442,899. It held that Advanz infringed the section 18 prohibition intentionally, or at the least negligently. The CMA divided the penalty between the various entities as follows: (i) Hg was liable for £8.6 million; (ii) Cinven was liable for £51.9 million; and (iii), Advanz Pharma Corp was liable for £40.9 million (after adjustment to prevent the penalty exceeding the statutory maximum).
B. An overview of the case
6. Given the substantial complexity of the facts I set out below an overview of the case.
Liothyronine Tablets
7. The drug in question is Liothyronine. It is used to treat patients with a thyroid hormone deficiency. The majority of patients suffering from hypothyroidism are treated with Levothyroxine which is the frontline drug. Liothyronine tablets are a second line treatment for those patients for whom Levothyroxine is ineffective. The tablets were developed in the UK in the mid-1950s and were sold under the brand name “Tertroxin”. Advanz acquired Tertroxin, which was then long off-patent, in 1992, as part of a portfolio of 22 products acquired by Goldshield Pharmaceuticals Ltd from Medeva plc for a consideration of £1 million. Advanz sold Tertroxin until 2007. The tablets are difficult to manufacture due to the low amount of active pharmaceutical ingredient per tablet and the sensitivity of Liothyronine to minor changes in processing technology. Advanz outsourced manufacture to third-party contract organisations and distribution was outsourced to wholesalers and pre-wholesalers.
Increases in the price of generic Liothyronine
8. The case of the CMA was that, over the Infringement Period, 2009-2017, Advanz abused its dominant position by raising the price for a box of generic Liothyronine tablets to excessive and legally unfair levels. There were 63 progressive price increases across the period. In 2007 a box was being sold at £4.05. By January 2009 the price had increased to £20.48. By August 2012 the price had risen to £46 and by October 2015 to £190. By July 2017 a box was being priced at £247.87. The CMA position was that these price increases were unjustified and were distant outliers when compared against the prices of a range of relevant comparables. In 2017 two companies, Teva and Morningside, entered the market and the price of Liothyronine Tablets fell but not back to the low prices being charged before 2009. In December 2024, at the time of this case, NHS reimbursement prices were at c.£76 per box.
Fairness and Cost Plus
9. The test in law of whether a price charged by a dominant undertaking is abusive and unlawful is fairness, a protean concept the subject of a great deal of case law over the years at both the EU and UK level. There are various methods which can be used to determine what a “fair” price is. In this case the drug in question is long-off patent, there was no requirement for any real innovation, there was a fixed and predictable demand which was highly inelastic and did not go down as prices went up and there were high barriers to entry which served to protect the incumbent dominant undertaking from competition. The CMA considered that the appropriate way to determine a fair price was to apply the “Cost Plus” test. Under this the CMA calculates the cost of production together with a reasonable rate of return on capital. It then compares the resultant cost figure with the average selling price (“ASP”) to see whether the margins charged were excessive and, if so, whether the prices were unfair in themselves or by comparison with other products. It takes into account any considerations that might be relevant to the “value” of the drug.
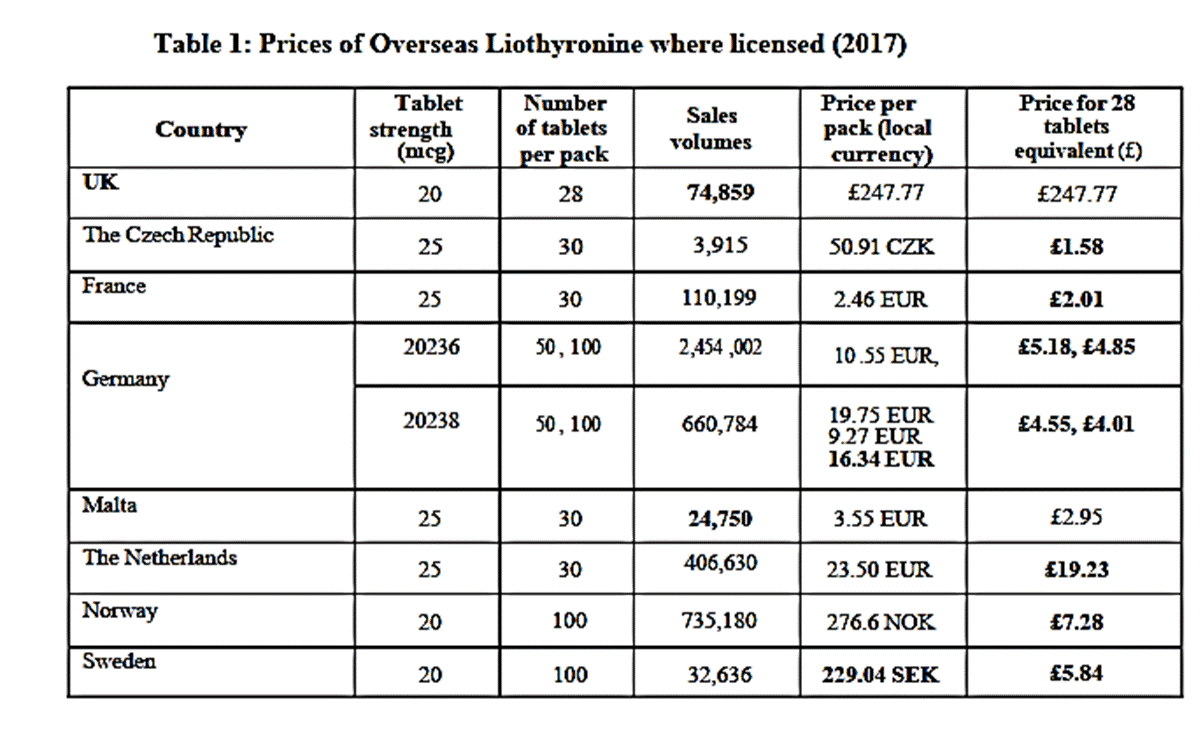
10. The CMA benchmarked the average Cost Plus figure it had arrived at for the Infringement Period of £4.94 per box against a variety of measures including: Comparable generic drugs referred to in a report on the generics market by economists, Oxera (“the Oxera Report”), prepared for the British Generic Manufacturers Association (“BGMA”) in 2019 and relied upon by the CMA before the CAT ; other drugs in the same category of the Drug Tariff with a similar market volume; NHS reimbursement prices across comparable drugs; and, prices for Levothyroxine tablets. It concluded that Cost Plus was appropriate in the context of these comparables. One especially telling comparator was the price of Liothyronine tablets sold overseas. In Judgment paragraph [73] the CAT found that “Advanz’s prices both during the Infringement Period and after the entry of Morningside and Teva were significantly higher than those prices.” Table 1 to the Judgment set out the details:
Alternatives to Cost Plus: The applicant’s theory of pricing
11. Applying the above process the CAT held that the differentials between Cost Plus and ASP were excessive and unfair in themselves and when measured against comparators. The CAT endorsed the CMA conclusion that the average cost, over the period, was £4.94 per box. The ASP however increased across the Infringement Period from a price close to Cost Plus, to £247. The CAT considered, but rejected, a range of alternative benchmarks put forward by the applicants. Whether the CAT was correct to reject these alternatives is at the heart of this case.
12. The applicants’ case can be summarised as follows. Where the structure of a market is conducive to effective competition regulators and courts should stay out. Judicial comment and economic literature indicate that where barriers to entry are surmountable markets self-rectify because high prices send signals to rivals encouraging new entry; they are a “magnet” drawing rivals into a market and creating competition where none might have existed before. The applicants cite the judicial commentary and the literature as highlighting the difficulties attached to ex-post regulatory enforcement which, it is argued, carries a substantial risk that intervention will cause more harm than good to consumers.
13. The applicants put forward a structural model of what it is argued amounts to a sufficiently effective, workably competitive, market. This comprises 3 conditions: absence of dominance; absence of collusion; and surmountable entry barriers. They contend that as from 2017, after the Infringement Period when new entry occurred, the market was characterised by the existence of these structural conditions. They then advance a series of discrete pricing tests or points which they say reflect the natural outcome of this workably competitive market and, that being so, the prices generated in such markets are necessarily fair and lawful. They say that it is possible to use these price points to extrapolate backwards in time in order to determine a series of benchmarks which can be used to evaluate the fairness of prices actually charged by Advanz during the Infringement Period. These price points are:
(i) Any price emerging from a market where there is no dominance, no collusion, and no insurmountable barrier to entry.
(ii) The price at which new entry is first incentivised.
(iii) The level at which, post new entry, prices settle.
(iv) The price that would be charged in a competitive market (involving multiple suppliers) where each undertaking’s costs would be split over lower market shares than if costs are spread over 100% of volume, with the result that the average cost and price will be higher.
(v) The (high) price actually charged, upon the basis that the resultant high profits can be used to subsidise prices in other product ranges. In the pharmaceutical sector this is said to represent good regulatory practice and be consistent with workable competition.
14. To meet the unhelpful but incontrovertible fact that these benchmark tests lead to much higher prices being treated as fair than Cost Plus, the applicants challenge the very basis of the Cost Plus test. It was said to be unreflective of economic reality and lacking in legal certainty. It was antithetical to good competition law policy because, by its nature, it curbed the charging of high prices which encouraged new entry and the emergence of new competitive market forces. It thereby entrenches market power and dominance. High prices were systemically good for the creation of healthy competition and thereby for consumers; Cost Plus was inimical to the development of new and healthy competition, it protected dominance, and was bad for consumers.
The legal basis for the applicant’s theory of pricing: workable competition
15. The applicants dress this theory in legal clothing by reference to the supposed endorsement in case law of the principle that prices are deemed to be fair, and hence lawful, where they reflect the conditions of “workable competition”. Where evidence of the prices that would be generated in a workably competitive market exists, it is argued that it is wrong in principle for regulators and courts to adopt a Cost Plus test.
16. The applicants contend that this approach is consistent with the test laid down by the Court of Appeal in CMA v Flynn Pharma [2020] EWCA Civ 339 (“Phenytoin”) which pulled together and summarised nearly 50 years’ worth of case law. Two central principles are said to underly the law as summarised in that case:
(i) That the overarching test is whether the dominant undertaking “… reaped trading benefits which it could not have obtained in conditions of normal and sufficiently effective competition i.e. workable competition”: Paragraph [97(i)].
(ii) That regulatory intervention can, perversely, harm the normal process of competition by preventing the emergence of markets that would otherwise self-correct through new entry. The Court in Phenytoin is said to have established the principle that where entry barriers can be overcome (ie are surmountable) markets are self-correcting and intervention risks prolonging a monopoly situation by blocking efficient pricing signals which otherwise promote market entry and the advent of real competition. A belief in the vitality of market forces is bolstered by the well-established high likelihood of regulatory failure in the case of price regulation: Paragraph [104].
The acquiescence issue
17. On a different note, it is also contended that since the prices actually charged during the Infringement Period had been paid by the NHS subject to a system involving drug tariff scrutiny there was legal acquiescence which meant that the prices charged could not be unlawfully unfair and abusive.
The position of the CMA and the CAT on pricing issues
18. In the Decision the CMA rejected all the arguments put forward by the applicants. Before the CAT the applicants adduced new evidence in particular of pricing trends in the period following the Decision and current at the time of the appeal. That evidence addressed the theory of pricing described above. The CAT, taking account of the new evidence, dismissed the appeals and upheld the Decision. In particular it endorsed the CMA’s finding on Cost Plus as an appropriate benchmark for determining a workably competitive and fair price in generic pharmaceutical markets: Judgment paragraph [230] and the conclusions at paragraphs [347]-[350].
The issue about penalties
19. On the appeal to the CAT a portion of the financial penalty imposed by the CMA in the Decision was set aside. That component amounted to a significant increment added by the CMA to the basic penalty to reflect a statutory policy imperative seeking to create what is termed “specific deterrence” i.e. a sum intended to deter repeat offending by the undertaking in question. This is a sum over and above any component of the penalty designed to achieve “general” deterrence which is included as a message to the world at large not to engage in the condemned conduct - pour encourager les autres. The CMA says that the CAT erred when removing the element for specific deterrence because it misconstrued and/or misapplied the relevant statutory guidelines on penalties that it was required to have regard to.
C. The issues arising for determination.
20. A limited number of proposed grounds of appeal were initially before the Court. They evolved in the course of written and oral submissions. The CMA objected but, on balance, the pragmatic course is simply to deal with them. They break down into a series of issues:
Issue I - “Workable competition”: The minimum conditions necessary for a workably competitive market / workability as a bright line test of fairness.
Issue II - EIP: The relevance to fairness of Entry Incentivising Prices (“EIP”).
Issue III - PEP: The relevance to fairness of Post Entry Pricing (“PEP”).
Issue IV - New pricing evidence: The relevance and admissibility of new evidence of PEP sought to be adduced and relied upon by the applicants relating to price movements subsequent to the Judgment of the CAT.
Issue V - MFP: The relevance to fairness of the prices that would have been charged in a competitive market with multiple suppliers needing to recover their fixed costs over lower volumes (“multi-firm pricing” or “MFP”).
Issue VI - Portfolio Pricing: The relevance to fairness of pricing designed to generate high profits in relation to one product which could then be used to subsidise other products (“Portfolio Pricing”).
Issue VII - Acquiescence: The relevance to fairness of the fact that the NHS did not object to pricing subsequently found by the CMA to be abusive.
Issue VIII - The burden and standard of proof: Did the CAT disregard the burden and standard of proof in relation to the pricing issues?
Issue IX - Penalties for specific deterrence: The approach of the CAT when applying the statutory Guidance to the calculation of penalties designed to create a specific deterrent to future repetition or infringement by the undertaking in question.
D. A summary of the relevant law
21. There is no real dispute about the law to be applied. I set out below a summary of: (i) the test of fairness under section 18 CA 1998; (ii) the nature of merits appeals before the CAT; and (iii), the jurisdiction of the Court of Appeal.
The test of fairness under section 18 CA 1998
22. Section 18 (1) CA 1998, entitled “Abuse of dominant position”, provides that any conduct on the part of one or more undertakings which amounts to the abuse of a dominant position in a market is prohibited if it may affect trade within the United Kingdom. Section 18(2) lists various examples of abuse. Section 18(2)(a) stipulates that conduct may amount to an abuse if it consists in:
“… directly or indirectly imposing unfair purchase or selling prices or other unfair trading conditions”.
23. There is no statutory definition of fairness in the CA 1998 or in equivalent treaty provisions at EU level. It is common ground that jurisprudence under the EU regime is relevant. At that level the locus classicus of the test of fairness is the judgment of the CJEU in Case C-27/76 United Brands v Commission EU:C:1978:22 (“United Brands”) in particular at paragraphs [248]-[253]. Markets exhibit an almost unlimited array of features and the evidence relevant to establishing abuse is commensurately diverse and variable. The Court in Phenytoin summarised the various approaches to evidence endorsed in case law. The underlying premise, which is reflected in the literature, is that no category or type of evidence is to be treated as necessarily dispositive or relevant, or indeed irrelevant. The guiding principle is weight, not admissibility: Phenytoin paragraphs [97(iii)-(vii)] and [105]. This means that all evidence is capable of being admitted but its value will be for the CMA and CAT, on an appeal, to weigh.
24. The judgment in United Brands, together with nearly 50 years of subsequent jurisprudence, was analysed by the Court of Appeal in Phenytoin (ibid) where at paragraph [97] the Court set out a summary:
“97. …
(i) The basic test for abuse, which is set out in the Chapter II prohibition and in Article 102, is whether the price is “unfair”. In broad terms a price will be unfair when the dominant undertaking has reaped trading benefits which it could not have obtained in conditions of “normal and sufficiently effective competition”, i.e. “workable” competition.
(ii) A price which is “excessive” because it bears no “reasonable” relation to the economic value of the good or service is an example of such an unfair price.
(iii) There is no single method or “way” in which abuse might be established and competition authorities have a margin of manoeuvre or appreciation in deciding which methodology to use and which evidence to rely upon.
(iv) Depending upon the facts and circumstances of the case a competition authority might therefore use one or more of the alternative economic tests which are available. There is however no rule of law requiring competition authorities to use more than one test or method in all cases.
(v) If a Cost-Plus test is applied the competition authority may compare the cost of production with the selling price in order to disclose the profit margin. Then the authority should determine whether the margin is “excessive”. This can be done by comparing the price charged against a benchmark higher than cost such as a reasonable rate of return on sales (ROS) or to some other appropriate benchmark such as return on capital employed (ROCE). When that is performed, and if the price exceeds the selected benchmark, the authority should then compare the price charged against any other factors which might otherwise serve to justify the price charged as fair and not abusive.
(vi) In analysing whether the end price is unfair a competition authority may look at a range of relevant factors including, but not limited to, evidence and data relating to the defendant undertaking itself and/or evidence of comparables drawn from competing products and/or any other relevant comparable, or all of these. There is no fixed list of categories of evidence relevant to unfairness.
(vii) If a competition authority chooses one method (e.g. Cost-Plus) and one body of evidence and the defendant undertaking does not adduce other methods or evidence, the competition authority may proceed to a conclusion upon the basis of that method and evidence alone.
(viii) If an undertaking relies, in its defence, upon other methods or types of evidence to that relied upon by the competition authority then the authority must fairly evaluate it.”
25. The central issues in this case relate to the legal relevance of, and the evidential weight to be attached to, different types of evidence relating to pricing. The applicants argue that the pricing evidence they adduced before the CAT was of such high probative value that it massively outweighed other categories of evidence relied upon by the CAT. A facet of this concerns the burden placed upon undertakings under investigation to raise issues and evidence which then triggers a legal duty on the regulator or court to evaluate that evidence (cf Phenytoin paragraphs [97(vii) and (viii)] above). In the present case this is significant because the CAT found a violation by Advanz on the basis of two categories of evidence (Cost Plus and comparables based upon current pricing) that, according to the settled jurisprudence, suffice in law to establish abuse and in respect of which there is no challenge in this Court. How does the evidential burden on a defendant undertaking operate in such a case?
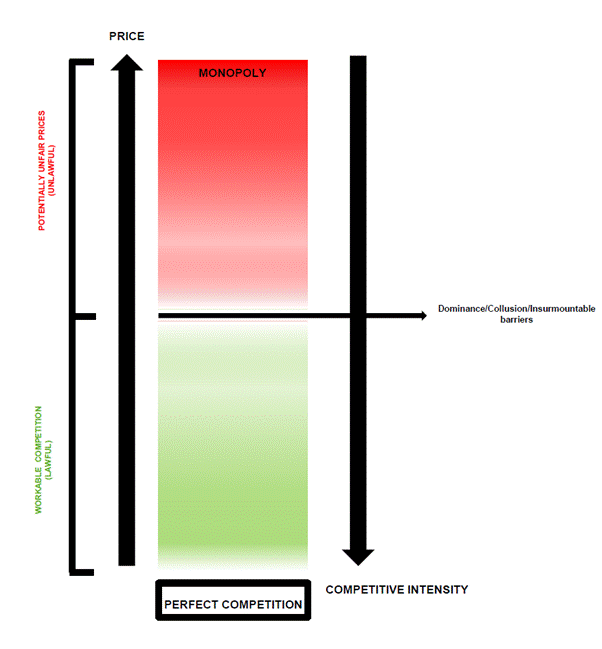
26. A second issue concerns the extent to which the relevance and weight to be attributed to various types of evidence are affected by legal principle. The applicants have referred to two types of legal principle. The first relates to the principles governing the concept of abuse. The second concerns more general principles of law. In relation to the former (abuse) the applicants say that because of the legal test for abuse particular types of evidence (in particular those they rely upon) constitute mandatory benchmark tests which are dispositive of the outcome. Where such evidence exists, it is unlawful for the decision maker to rely upon other types of evidence. In relation to the latter (general principles) the main example referred to concerns the principle of legal certainty which was relied upon by the applicants to support their argument that certain types of evidence should be accorded greater or lesser weight than other types. For instance, the applicants contend that their evidence of settled prices (in the context of Issue III - PEP) is superior as a test in law in terms of legal certainty to evidence of stabilised prices which are free from contamination as a test, and that the CAT erred in law in attaching greater weight to this latter category of evidence. For reasons set out below legal certainty is a well-established principle of law which has been applied to determine the value of different categories of evidence: see paragraphs [142]-[145] below which explain that, because of the principle, evidence that is available to a dominant undertaking when it sets prices (such as its own costs) is more informative of whether there is abuse, than evidence which the undertaking could not have been aware of at the relevant time.
The nature of merits appeals before the CAT
27. The hearing before the CAT was an appeal on the merits of the CMA Decision. It was not a judicial review. The CAT admitted and took account of new evidence not before the CMA. It endorsed fully the findings of fact and reasoning in the Decision but also made some limited independent findings of fact. The CAT summarised its jurisdiction, by reference to the analysis in Phenytoin:
“121. The fourth main issue on the appeal [in Phenytoin] was as to the extent to which the Tribunal was bound by the CMA’s margin of manoeuvre or discretion in exploring factual matters. Green LJ noted that the CMA had a “margin of manoeuvre” (the terms used by the Court of Justice in Latvian Copyright) or “appreciation” or “discretion” which flowed from the fact that the legal test under Section 18(2)(a) CA 1998 and Article 102(a) is broad brush and necessarily confers a significant latitude upon a competition authority as to the methods and evidence bases that it resorts to in order to prove an abuse of unfair pricing. He continued as follows:
“136. But this is quite different in principle to the question whether the Tribunal, as a supervisory judicial body, must pay deference to that exercise of judgment. Under the CA 1998 the Tribunal has a merits jurisdiction as to both law and fact and upon the basis of established case law it is not bound to defer to the judgment call of a competition authority. It is empowered under the legislation to come to its own conclusions on issues of disputed fact and law and can hear fresh evidence, not placed before the CMA, to enable it to do so.”
122. Green LJ held that the conferral of a merits jurisdiction upon the Tribunal flows from important legal considerations relating to the rights of defence and access to a court, under fundamental rights such as Article 6 of the European Convention on Human Rights, competition law being treated as a species of criminal law as a recognised in numerous cases. Green LJ summarised the case law as follows.
“140. From case law it is possible to draw various conclusions about the role of judicial bodies in relation to the margin of appreciation of a competition authority: (i) for a (non-judicial) administrative body lawfully to be able to impose quasi-criminal sanctions there must be a right of challenge; (ii) that right must offer guarantees of a type required by Article 6; (iii) the subsequent review must be by a judicial body with “full jurisdiction”; (iv) the judicial body must have the power to quash the decision “in all respects on questions of fact and law”; (v) the judicial body must have the power to substitute its own appraisal for that of the decision maker; (vi) the judicial body must conduct its evaluation of the legality of the decision “on the basis of the evidence adduced” by the appellant; and (vii), the existence of a margin of discretion accorded to a competition authority does not dispense with the requirement for an “in depth review of the law and of the facts” by the supervising judicial body.”
123. Green LJ went on to note that the conferral of a merits jurisdiction did not mean that the jurisdiction of the Tribunal is unfettered. The Tribunal should interfere only if it concludes that the decision is wrong in a material respect. Whether an error is material will be a matter of judgment for the Tribunal. The Court of Appeal dismissed the CMA’s appeal against the Tribunal’s finding that the CMA had conducted an insufficient examination of evidence of comparators and its appeal against the Tribunal’s conclusion that the CMA had failed to take proper account of patient benefit in its assessment of “economic value” as that phrase is used in paragraph [250] of United Brands. It was open to the Tribunal to reach a different conclusion to the CMA on these matters.”
The jurisdiction of the Court of Appeal
28. For an appeal to be mounted either the permission of the CAT or that of the Court of Appeal is required. The test in CPR rule 52.6(1) applies. Accordingly, permission may be granted where either the CAT or the Court of Appeal considers that the appeal would have a real prospect of success or there is some other compelling reason for the appeal to be heard. The substantive jurisdiction of the Court of Appeal is governed by section 49(1) CA 1998 which limits substantive appeals to points of law but imposes no equivalent limitation in appeals concerning penalties:
“An appeal lies to the appropriate court—
(a) from a decision of the Tribunal as to the amount of a penalty under section 36;
(b) …
(c) on a point of law arising from any other decision of the Tribunal on an appeal under section 46 or 47.”
E. Liothyronine pricing
29. The nub of the applications concerns issues relating to principles of pricing. The position is complex. It is necessary, by way of introduction, to explain the issues and to provide a summary of the position as it stands for the purposes of the case before the Court.
30. The target of a merits appeal is the CMA Decision. In this case, after what was in effect a trial, the CAT upheld the Decision on dominance and abuse in all respects. The Decision is therefore the starting point. It is a lengthy document. The substantive content runs to 433 pages and is accompanied by annexes exceeding 200 pages which include the detailed workings of the CMA in relation to the computation of Cost Plus. The CAT summarised the Decision in Section C of the Judgment (paragraphs [80ff]).
31. Three measures or benchmarks of price are important to the CMA Decision and the CAT Judgment:
(i) The first is Cost Plus where the average across the Infringement Period was found to be £4.94. This finding made in the Decision was endorsed by the CAT.
(ii) The second is £20.48 which was the ASP prevailing in the market as of the commencement of the Infringement Period in January 2009. This was a finding by the CMA and was unchallenged before the CAT.
(iii) The third is £21 which is the price the CAT found was a viable entry inducing price or EIP. There was no equivalent finding to this effect in the CMA Decision. It is a finding the applicants challenge before this Court.
Cost plus (£4.94) / comparison with the ASP
32. The CAT having received full evidence and formed its own independent conclusions (Judgment paragraph [149]), agreed with the analysis of the CMA on Cost Plus: Judgment paragraphs [142]-[230] and [347]-[350]. It specifically upheld (cf paragraphs [230] and [348]) the conclusion of the CMA that the prices charged by Advanz during the Infringement Period above Cost Plus were excessive and abusive. There is no challenge to the calculation by the CAT of the applicable Cost Plus. I summarise the position briefly by reference to the reasoning and findings in the Decision.
33. The Decision sets out conclusions on Cost Plus in Section 5 (paragraphs [5.102] - [5.202]), and additional workings are set out in Annex 3 (“Costs plus a reasonable rate of return”) and Annex 4 (“Cost of capital”).
34. In relation to the cost element of Cost Plus the CMA first considered the cost of production. The CMA split the total costs involved in supply into direct and indirect costs and a reasonable rate of return. Without descending into detail, the CMA attributed values to: direct costs per unit; indirect and common costs per unit; amortisation charges; depreciation charges; return on intangibles; return on tangibles; and, return on working capital (see Decision page [197] Table 5.1).
35. In relation to the plus element of Cost Plus this is dealt with in the Decision at paragraphs [5.126ff] under the heading “Reasonable rate of return”. The CMA explained that it was normally necessary to allocate a reasonable rate of return to cover the cost of capital. The reasonable rate of return reflected the opportunity cost to investors of providing capital to Advanz to purchase assets and fund working capital requirements. The CMA applied a return on capital employed (“ROCE”) model.
36. The CMA also applied a series of “sensitivities” to Cost Plus relating to: common cost allocation; the approach to product rights valuation; and a reasonable return on capital bracket (i.e. WACC) which were calculated to be “very favourable to the parties”: (Decision paragraph [5.176]). In Table 5.2 (Decision page [201]) it set out its base line average figure for Cost Plus (£4.94) and an adjusted figure to take account of these favourable sensitivities (which led to a simple average of £7.35).
37. The CMA’s conclusion on Cost Plus was cross-checked against a significant number of comparables which the CAT analysed and heard evidence about. It held that: “No adequate explanation was given by the Appellants for Liothyronine Tablets’ outlying status in these comparisons”: Judgment paragraph [277].
38. The CMA addressed, and rejected, alternative measures of a fair price advanced by the parties which posited a much higher price level at which to set the fairness bar: See Decision paragraphs [5.195ff]. These included: post-entry prices; the price of other products similar to Advanz’s Liothyronine Tablets; portfolio pricing; forecast prices; the implications of Cournot modelling; entry plan pricing; and multi-firm pricing.
39. The CMA also considered whether there were other factors which justified taking a higher price as the threshold for fairness. It considered and rejected: the existence of demand side factors which added to the economic value of the product; whether the characteristics of the product could be expected to create enhanced value for consumers; the therapeutic value of the product to consumers; whether customers were willing to pay a premium for the product; whether the prices paid reflected substantial market power; and whether the prices charged were the outcome of agreement between Advanz and the NHS.
40. The CMA’s case was that Liothyronine prices in a competitive generics market should be close to, but typically slightly above, production costs. They would be at or proximate to Cost Plus: see Decision paragraphs [5.285(a)(iii)] and [5.292]-[5.301]. The CAT endorsed this conclusion: e.g. Judgment paragraph [348].
41. In paragraph [5.177] and Table 5.3 the CMA set out conclusions on the differential between the ASP and both the base line Cost Plus and Cost Plus adjusted for sensitivities in the Infringement Period 2009 - 2017. In Figure 5.8 (Decision page [202]) it produced the same information in graphic form showing how Advanz’s ASP had risen relative to Cost Plus (with/without sensitivities) over the Infringement Period. This showed that even the more undertaking-friendly Cost Plus with sensitivities made no real difference to the analysis.
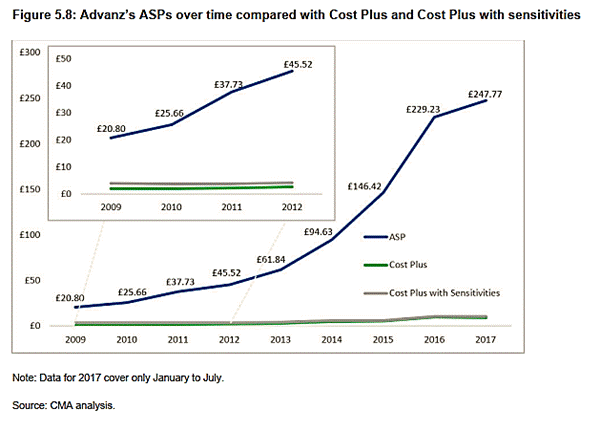
42. Prices charged for Liothyronine were substantially higher than Cost Plus. In 2009 (the first year of the Infringement Period), the differential was 900% per pack increasing to over 6000% at other points relative to September 2007. Figure 1.1 in the Decision tracks the ASP from January 2007 (before the Infringement Period) to July 2017. Paragraphs [1.09] and [1.10] stated:
“1.9 In October 2007, Advanz began applying this strategy to Liothyronine Tablets. At that time, the price of Liothyronine Tablets was £4.05 per 28 tablets and Liothyronine Tablets were already one of Advanz’s top ten most profitable products.
1.10 Advanz removed the ‘Tertroxin’ brand, re-launched Liothyronine Tablets as a generic product, and immediately implemented a price increase. As a result, Advanz nearly doubled the price of the drug overnight. Within a year of de-branding, Advanz had more than doubled its price again and by January 2009, its average sales price (‘ASP’) for Liothyronine Tablets had reached £20.48. Under HgCapital’s ownership (December 2009 to August 2012), the ASP of Liothyronine Tablets increased from nearly £21 per pack to nearly £46 per pack; under the Cinven Entities’ ownership (August 2012 to October 2015), this increased again to nearly £190 per pack. By July 2017, nearly 10 years after de-branding, Advanz had increased the ASP of Liothyronine Tablets from £4.05 to £247.87, representing a price increase of 6,021% since September 2007.”
The price prevailing at the commencement of the Infringement Period (£20.48)
43. I turn now to the figure of £20.48. For reasons of administrative priority, and in accordance with its published policy, the CMA did not formally set average Cost Plus (£4.94) as the ceiling price above which, as a matter of enforcement policy, the Decision was to be predicated. Further, it did not use 2007 as the starting point for the Infringement Period. Instead, it adopted the more conservative (and hence pro-undertaking) figure of £20.48. This was the price at which Advanz was selling the tablets in January 2009 which then marked the commencement of the Infringement Period: see Decision paragraph [1.10] cited above.
44. In relation to the CMA’s conclusion that prices above £20.48 were excessive, and not objectively justified, the CMA set out detailed reasons. The analysis is found in the Decision between paragraphs [5.252]-[5.276] and a summary is set out in paragraph [5.251]:
“5.251 In coming to this conclusion, the CMA has had regard to the following factors:
(a) The substantial disparity between Advanz’s prices and the economic value of its Liothyronine Tablets;
(b) The competitive conditions prevailing during the Infringement Period, including the absence of alternative Liothyronine Tablet suppliers, lack of regulatory constraint, high demand inelasticity, high barriers to entry and lack of countervailing buyer power, enabled Advanz to sustain prices which bore no relationship to economic value;
(c) The commercial purpose of Advanz’s pricing strategy, which was to exploit the lack of competitive pressure on its pricing resulting from the competitive conditions set out at (b) above;
(d) The increases in price were significant, amounting to a 6,021% increase in Advanz’s prices (from £4.05 to £247.87) between the decision to de-brand and Advanz’s highest price; and a 1,110% increase over the Infringement Period (from £20.48 to £247.87), with no material increase in production costs or innovation;
(e) Advanz’s price increases have had a significant adverse impact on the NHS and patients; and
(f) There is no independent or objective justification for the conduct.”
45. The CMA left open the question whether a pre-2009 price above Cost Plus but below £20.48 was excessive and unfair as a matter of law. Paragraph [5.105] of the Decision explained:
“Taking account of its prioritisation principles, the CMA decided to focus its Investigation only on prices of £20.48 per pack (the price in January 2009) and above. The CMA has not reached a conclusion on the exact level (above Cost Plus but below £20.48 per pack) at which Advanz’s prices became excessive and unfair as a matter of law. Therefore, although it is possible that prices somewhere above Cost Plus but below £20.48 per pack may have also been excessive and unfair, the CMA has limited itself to finding that Advanz’s prices were excessive and unfair when they reached at least £20.48 per pack. This means that the lowest price which is covered by the CMA’s infringement finding exceeds Cost Plus by 900% for the year 2009.”
In footnote [2] the CMA reiterated: “The CMA has decided for reasons of administrative priority not to pursue its investigation in respect of Advanz’s conduct during the period from 1 November 2007 to 31 December 2008 or following 31 July 2017. See Prioritisation principles for the CMA (CMA16), dated April 2014.” In footnote [1684] the CMA confirmed that £20.48 was the price charged in January 2009 and stated that prices below that level may also have been excessive and unfair. It added a caveat in footnote [828] where it made the important point that: “Cost Plus already includes a reasonable rate of return. However, as set out in paragraphs 5.65 ff above, not every price above Cost Plus would have been excessive and unfair.” For example, in paragraph [5.87] the CMA, in relation to the concept of “value”, observed in general terms: “The economic value of a product may exceed Cost Plus as a result of non-cost related factors including, where applicable, ‘additional benefits not reflected in the costs of supply’ or any ‘particular enhanced value from the customer's perspective’”.
46. The CMA, for the same administrative reasons, fixed the end of the Infringement Period as 31 July 2017 which was shortly prior to the new market entry of Morningside and Teva. It therefore left open whether Advanz was dominant after the end of the Infringement Period and, if so, for how long. The CMA explained that its calculation of penalties was conservative because it ignored the possibility that prices were excessive and unlawful both before and after the Infringement Period:
“7.134 To calculate the minimum direct financial benefit for each ownership period, the CMA has calculated the difference between its ‘enforcement price’ of (£20.48), that is the lowest price charged for Liothyronine Tablets during the Infringement Period that has been found to be excessive and unfair and Advanz’s actual selling prices during each of the different ownership periods of the Infringement. The resulting figures are then multiplied by the volumes sold in each ownership period. This results in a conservative estimate since profits based on prices that were lower than £20.48 could also be unlawful; the calculation also does not take into account any potential excess profits based on prices charged following the end of the Infringement Period.”
47. Before the CAT the applicants did not, for obvious reasons, challenge the limitation upon the scope of the Decision introduced for administrative reasons. But they did attack the CMA finding of £4.94 as a fair and lawful price and the endorsement of that conclusion by the CAT which was clear that a price above Cost Plus was excessive and unfair.
A viable entry inducing price (£21)
48. The third price of importance is £21. In evidence before the CAT the expert for the CMA, Professor Valletti, opined that Uni-Pharma (a company with experience of selling in the Greek market) made a serious attempt at market entry in 2010 (Judgment paragraph [300]). The evidence on Uni-Pharma was set out in the Decision and was limited to the reason why Uni-Pharma decided ultimately not to pursue its Marketing Authorisation (“MA”) application. The CMA considered generally MA applications made by a variety of third parties and in particular companies that had applied where the application process was ongoing at the time of the Decision (and who had not therefore then entered the market) or who had withdrawn their applications rather than engage in further investment to obtain the MA: see Decision paragraphs [3.99ff]. More specifically, the CMA considered the position of Uni-Pharma which had initiated the application process, but which had then withdrawn citing the costs of carrying out certain studies required by the HMRA as the cause: see e.g. Decision paragraphs [3.110], [4.139] and [4.142].
49. There was a dispute before the CAT on the evidence as to whether the then prevailing price was in fact considered by Uni-Pharma to be a viable entry price (Judgment paragraphs [300] - [301]). In paragraph [308], the CAT recorded the CMA argument that if (which it did not accept) entry inducing prices (EIP) were relevant then the attempt of Uni-Pharma:
“…to enter the UK and Irish markets, which began in 2010, was a credible entry attempt. The CMA accepted that it is not possible to determine whether Uni-Pharma withdrew only because its API manufacturer had withdrawn. It was, however, clear that an experienced manufacturer of hypothyroidism medicines took significant steps towards entry, including through the preparation of a dossier based on an API by a manufacturer in the market and spending €350,000. That was a credible, even if unsuccessful attempt at entry. In the circumstances, the price of £21 at which Uni-Pharma’s entry was sparked (March 2010) would be the relevant benchmark for an Entry Incentivising Price”.
50. The CAT (Judgment paragraphs [317ff]) concluded that the CMA was justified in rejecting EIP as a valid competitive benchmark. It was common ground between the experts that EIP did not reflect the outcome of an effectively competitive market. However, the CAT then made an independent finding of fact which was that, on the alternative hypothesis that EIP were relevant, Uni-Pharma did consider £21 to be a viable entry price:
“325. Had we considered that Entry-Incentivising Prices were a useful benchmark, we would have taken the relevant Entry-Incentivising Price to be the £21 current in 2010 when Uni-Pharma commenced its entry attempt. Although it is not clear to what extent Uni-Pharma’s discontinuance was attributable to the discontinuance of the API or to the need to invest in the bioequivalence study, that price appears to have been considered by Uni-Pharma to be a viable price which merited significant work and costs.”
(emphasis added)
The position before the Court of Appeal
51. Cinven, Hg and Advanz Pharma Corp appealed the Decision to the CAT. They adduced evidence to show that the prices charged were fair when set against various comparators and other benchmarks. This included new evidence which post-dated the Infringement Period and the Decision and covered the period between the Decision and the appeal. The CAT dismissed the appeals. The CAT did however reduce the penalty on a particular ground relating to the need for specific deterrence and reduced the penalty on each appellant accordingly.
52. Cinven and Advanz now seek permission to appeal against the Judgment. They argue that the CAT erred in rejecting their pricing evidence and thereby wrongly found that the abuse was (ignoring administrative enforcement priorities which led to £20.48 being the ceiling for abuse) to be determined by reference to the Cost Plus test. The CMA seeks permission in relation to the reduction in the penalty imposed on Cinven. It does not appeal the equivalent reductions in relation to Advanz or Hg. As to Advanz any appeal would be academic because the penalty, even as reduced, would still be above and therefore subject to the statutory maximum. As to Hg it initially sought permission to appeal but it compromised its dispute with the CMA and this included any appeal by the CMA against the reduction in penalty to Hg.
53. The position before this Court can be summarised as follows. The CAT upheld various factual markers found by the CMA:
(i) Advanz was the sole supplier of Liothyronine Tablets during the period 1st November 2007 to 31st July 2017: Decision paragraph [1.2]. It held a dominant position in the market for Liothyronine Tablets: Judgment paragraphs [142(4)] and also [351]-[391] in relation to the absence of any countervailing bargaining power on the part of the NHS.
(ii) Advanz abused its dominant position by charging excessive prices in excess of Cost Plus which were unfair and abusive from 1st January 2009 to 1st July 2017 (the Infringement Period): Decision paragraph [1.4] and Judgment paragraphs [230] and [347]-[350].
(iii) The average Cost Plus across the Infringement Period was £4.94: Judgment paragraph [145].
(iv) Advanz first applied a price optimisation strategy in October 2007 (Decision paragraph [1.9]) at which point in time the price of Liothyronine Tablets was £4.05 per box of 28 tablets: Decision paragraph [1.9] and Figure 1.1 which show that the price had been c.£4 since at least the start of 2007. The strategy was designed to raise prices to their highest possible level whilst avoiding regulatory scrutiny: Judgment paragraphs [29]-[72].
(v) Between January 2009 and July 2017, the price was increased upon 63 occasions: Judgment paragraph [32].
(vi) The first point in time when prices went above average Cost Plus was October 2007 (when it rose to £8.05): Decision paragraph [3.190(b)] and Figure 3.2 .
(vii) As of July 2017, the price was c.£247 per box: Decision Table 1.1 page 10.
(viii) There were no objective, technical, safety or other considerations which justified the price increases: Judgment paragraphs [216], [321]. In a competitive market competition would (once initial fixed costs had been recovered) “drive prices closer to the direct costs of production”: Judgment paragraph [228(3)].
(ix) New entry to the market occurred in August 2017 (Morningside) and September 2017 (Teva) when the ASP was c.£247: Judgment paragraph [74]-[79].
(x) Without prejudice to whether Advanz still held dominance, market wide prices following the Infringement Period, causally, remained contaminated by the prior abuse: Judgment paragraphs [268] - [281].
(xi) Uni-Pharma considered £21 to be a viable entry price: Judgment paragraph [325].
54. In addition, the CAT did not: (i) disturb the decision of the CMA to choose a date of January 2009, and the then prevailing price of £20.48, as the start of the Infringement Period for administrative reasons; or (ii), disagree with the CMA that Advanz might have been dominant and acted abusively after the Infringement Period.
F. Key facts as found by the CAT
55. The facts are set out fully in the Judgment. I set out below a summary of the matters of greatest relevance to the applications before this Court. Those concern: (i) The pricing regime; (ii) the strategy of Advanz behind the pricing of Liothyronine tablets to the NHS; and (iii), post-Infringement Period entry into the market.
The pricing regime
56. Branded drugs are subject to price regulation pursuant to a voluntary scheme agreed between the DHSC and the Association of the British Pharmaceutical Industry. During the Infringement Period this regulation occurred via a voluntary arrangement known as the pharmaceutical pricing regulation scheme ("PPRS") which applied to manufacturers and suppliers of branded medicines to the NHS. Advanz was a member of the scheme. However, since Liothyronine Tablets were unbranded after 2007, the PPRS did not apply during the Infringement Period.
57. The cost of prescriptions for generic drugs is funded via a reimbursement price paid to dispensing pharmacies for completing NHS prescriptions. The reimbursement price is set out in a list known as the Drugs Tariff (“DT”) published upon a monthly basis by NHS Prescription Services on behalf of the DHSC. Drugs covered by the Drug Tariff are allocated to one of three categories: A, C or M. These determine the price for the product. Category M applies to commonly used generic drugs available from several sources. Category A drugs must be listed either by two wholesalers or by one wholesaler and by two manufacturers. Between December 2007 and November 2010 Liothyronine Tablets were not included in the Drug Tariff and the price paid to Advanz by the NHS was the list price. From November 2010 to April 2015 the tablets were listed in category A of the Drug Tariff. In May 2015 they were moved to category C where they remained until March 2018 when they returned to Category A. In January 2019 the tablets were moved to Category M.
58. During the Infringement Period Scheme M was a voluntary scheme concluded between the Secretary of State for Health and the BGMA. During the Infringement Period the pricing of Liothyronine Tablets was subject to Scheme M. It applied to manufacturers and suppliers of generic drugs sold to the NHS and it permitted members to alter the price at which medicine was sold to wholesalers or dispensing contractors without any requirement to discuss such changes with the NHS in advance. Members notified price changes to the NHS and they would be paid. The provisions of Scheme M specified that the DHSC could intervene to ensure that the NHS paid a reasonable price for the drug if it appeared that normal competitive conditions were not operating so as to protect the NHS from significant increases in expenditure.
The strategy of Advanz behind the pricing of Liothyronine tablets to the NHS
59. In 2007 Advanz de-branded the product and thereafter sold it as a generic. It amended the MA to remove the branding and gave the drug its generic name as set out in British Pharmacopoeia (“Liothyronine”). The decision to de-brand was part of a strategy by Advanz to increase profitability through price increases. The Decision records how this strategy evolved over time as evidenced in internal documents. There is no challenge to inferences drawn from this material. It is said though that whilst it might well convey a pejorative feel or tone, this has no bearing upon any issue relevant to the allegation of abuse. I agree that it is necessary to be careful not to be swayed by the tone of the exchanges. Businessmen sometimes use flamboyant and provocative language (“let’s kill the competition”) which can be ambiguous and open to a number of meanings. Nonetheless, that does not mean that documents relating to strategy lack relevance where they provide evidence and information such as how competition in the market operates, the supplier’s strategy, and provide a benchmark against which claims that the pricing strategy was objectively justified can be measured. The CMA and CAT were correct to treat internal documents which evidenced intent as capable of having probative value. See further paragraph [123] below.
60. By de-branding, products were removed from the PPRS and from price regulation. The CAT (Judgment paragraphs [31] and [32]) summarised the policy as it stood in 2007:
“31. The decision to de-brand was part of a strategy by Advanz to drive an increase in profitability through price increases. By de-branding, products were removed from the PPRS scheme and hence from price regulation, as recognised in Advanz’s UK business plan for branded pharmaceuticals in April 2007:
‘The way in which the PPRS scheme works means that price increases cannot be made easily on branded products. In order to drive price increases there is a strategy to move to the generic name and increase prices.
…
A range of products can be moved from branded to generic resulting in their removal from the current PPRS scheme and hence from price regulation. Prices on these products can be increased.’
32. John Beighton, Advanz’s former CEO, speculated in his witness statement about other possible reasons for the decision to de-brand (a decision which was taken several years before he joined Advanz), including the obsolescence of the Tertroxin brand name but these are not reflected in the contemporaneous documents. Advanz proceeded to implement a series of price increases in accordance with this strategy. Immediately prior to the de-branding of Tertroxin in October 2007, the average selling price (“ASP”) for the drug was the equivalent of £4.05 per 28 tablet pack. It was Advanz’s seventh most profitable product in its portfolio of 62 drugs. Having de-branded the drug, Advanz reduced the pack size from 100 to 28 and immediately increased its ASP to £8.05 per pack, in effect nearly doubling the price. A series of 63 individual price increases followed …”
61. The CAT cited from a due diligence report prepared by McKinsey and Company prepared for Hg which acquired the business in 2009 as part of a management buy-out and which highlighted the inadequacy of competitive pressures as an explanation for the “extraordinary” success of the strategy of exploiting niche generic drugs:
“33. The reason behind the extraordinary success of the pharmaceutical division in the difficult generics market in the UK is the efficacious management of its product portfolio within the regulation schemes in the UK: Trojan (i) manages to position its products in niches where competition is absent or very limited, (ii) optimally manages their products within the regulatory pricing schemes (branded and non-branded). Often their sales level stays under the radar screen of potential new entrants, thus protecting their business.”
62. Additional disclosed material (e.g. reports and presentations) from the time of the subsequent sale to Cinven in 2012 addressed: whether significant price increases would have a negative effect on volumes; the absence of competitors; the high barriers to new entrants; and the favourable regulatory environment. A report in May 2012 from IMS Consulting Group entitled “Project Glacier Final Report” suggested that a price of £60 per pack was sustainable. This highlighted that there was an off the radar “niche” in the market which sat between the commercial ranges of small generic companies and large players in which the applicants would be uniquely well positioned. The report referred to: “…niche products that fall under the radar of large players but above the size threshold of small generic companies. Furthermore, these products are difficult to manufacture thereby reducing the risk of new competitors.” Other internal documents refer to this niche as not being economically viable for new entrants to invest resources in to develop competing products.
63. This niche was also immunised from the risk of regulatory intervention. Documents refer to the: “… particularly beneficial reimbursement mechanism which, whilst effective for high volume products which is what the NHS cares about does allow for niche players to achieve good margins”. An Investment Recommendation submitted to the Cinven Investment Committee on 2nd July 2012 included the following:
“Reimbursement for drug manufacturers is controlled by a small group within the DoH, who aim to minimise the NHS’ £11bn drug bill whilst ensuring drug availability The focus is on high volume drugs (patent and off-patent) as this is where the absolute quantum of savings is higher: niche products are typically below the radar […]
Some of Mercury's products display price inelasticity, with no volume response from successive price increases.”
And later:
“Mercury therefore operates below the radar and capitalises on opportunities to achieve volume and pricing growth even in such a heavily regulated market.”
64. An illustration of the way in which price inelasticity and the absence of regulatory oversight for therapeutically important drugs played out was described in an internal email dated 19th July 2012. This indicated that the price optimisation strategy applied to Liothyronine was applied to other drugs as well:
“All these products are life saving products and exclusively marketed by Mercury Pharma only. There is no other substitute in UK market for these products. After de-branding ... we have increased the prices continuously in last 3 to 4 years. We have also changed the pack sizes of the products without reducing the prices. Few of the examples are like ... Liothyronine, where we have reduced the pack size from 100 to 28 ... we could continue increasing the prices [year on year] subject no other company introduces these molecules. Since these are de-branded therefore they do not have any PPRS liability also.”
65. The Decision (paragraph [5.257]) records that a Cinven Partner observed in July 2012, shortly before Cinven’s acquisition of the Advanz business from Hg, that what drove generic prices upwards was oligopolistic market structures, not demand growth:
“… the business’s ‘primary “tail wind” is price increases passed on the payor because of the oligopolistic nature of most segments it operates in, rather than a real growth in volume for each drug’ and the business model relied upon the ‘European healthcare systems … under very strong pressures [not reacting because] “it is too below the radar screen/noise”.”
66. The Judgment (paragraphs [46]-[53]) cites a slide pack presentation given by Mr Beighton to lenders (September 2012), shortly after Cinven’s acquisition of the business. This shows that for off-patent products there was no research spend, strong barriers to entry and pricing power. The CAT cites from accompanying speaking notes. Examples include:
“Attractive position - niche off-patent products insulated from key pharma risks -
No R&D spend or patent cliff -
Little/no competition - pricing/margin power -
Strong entry barriers mean position sustainable.”
Another slide entitled “Differentiated product portfolio benefits from high barriers to entry” highlighted barriers to entry:
“Manufacturing Process
• Products require complex manufacturing process and have difficult to determine formulations
Regulatory Approval
• Competitors entering market need to obtain new marketing authorisations
• Process is costly and can be time-consuming (c.3-4 years)”
67. The existence of high entry barriers does not preclude the possibility that ultimately they are surmountable. If the prevailing price is very high, there might come a time when potential competitors are incentivised to incur the burdens and costs of overcoming the barrier in order to exploit the high price on the other side. The CAT found (Judgment paragraph [61]) that there was appreciation from May 2013 onwards on the part of Advanz that if prices continually went upwards, entry was to be expected. It was foreseen that this pricing strategy could provoke entry relatively soon. The plan was to maintain price increases including before the anticipated generic entry. Advanz should, opportunistically, “take what it can now”. The CAT observed:
“A presentation document headed “UK key molecules” forecast loss of volumes in 2017 offset by increased prices, achieving year on year revenue gains in the period 2015 to 2018. This was consistent with the strategy adverted to in an email dated 31 May 2013, some two years earlier, in which Mr Beighton advocated a price increase in relation to another drug (Prednisolone):
“… because I am pretty sure that we are going to get competition within the next year or so. I know of at least [one other supplier] that are developing. Therefore we should take what we can from it now. I think Liothyronine may be a similar story…”
68. Other documents refer to there being no need for innovation because the drug had long established “strong efficacy and safety” (Judgment paragraph [64]).
69. By 2016 there was press commentary focusing upon the increase in tablet prices and predicting an adverse NHS response. The CAT observed that this had no impact upon sales:
“67. The price increases also attracted adverse scrutiny in the press. An article in the Times dated 5 June 2016 reported that doctors had been encouraged to stop prescribing Liothyronine after the price of a tablet shot up from 16p to £9.22. The article was forwarded within Advanz, prompting concern that the change of guidance might impact on sales. In response to an internal enquiry as to what was meant by the reference in the article to the NHS encouraging doctors to stop prescribing Liothyronine, and whether there would be a big impact, the answer was as follows:
“Business as usual. We have seen a very small volume decline over the last 18 mths but it is very small (1-2%). So we characterise the market and volumes as flat!”
68. A subsequent internal email dated 29 June 2016 commented as follows: “[…] In short -- nothing new. The most important thing about this is the date. [The] ... DROP-List is published every year. It was published a year ago and our volumes remain flat. Thus, it has had no impact on the sales volumes.”
70. Ultimately, the continued upward trajectory of prices did trigger regulatory intervention (Judgment paragraphs [71] and [72]). In 2017 an NHS Clinical Commissioners consultation occurred which led to guidance being issued to CCGs to reduce the prescribing of Liothyronine Tablets on cost grounds. As a result, some patients previously prescribed with Liothyronine Tablets had their treatment withdrawn. Some were able to obtain supplies privately. Following the publication of an adverse Times article (June 2016), Jeremy Hunt, the then Secretary of State for Health, asked the CMA to look into whether drugs companies had been guilty of excessive pricing.
Post Infringement Period entry into the market
71. Advanz was the only holder of an MA for Liothyronine Tablets throughout the Infringement Period which ran to the end of June 2017, at which point in time the ASP was at about £247 per box. Subsequently, a number of MAs for Liothyronine Tablets have been granted. The first was to Morningside in June 2017. It commenced development in 2012 and submitted its application for an MA in July 2015. This resulted in a number of deficiency letters from the MHRA. Morningside considered that “the process for obtaining [an MA] was … challenging”, despite receiving “tremendous support from the MHRA.” Morningside ultimately commenced supplying Liothyronine Tablets on 21 August 2017. On 14 August 2017, the MHRA granted an MA to Teva. It first contacted the MHRA regarding an MA application in November 2014 and submitted an application in December 2016. It began supplying Liothyronine Tablets at the end of September 2017. Accord-UK, initiated a development project for Liothyronine Tablets in 2012 and submitted an MA application in June 2020. Sigmapharm submitted an application in 2019. Both now have an MA.
G. Issue I - “Workable competition”: The minimum conditions necessary for a workably competitive market/Workability as a bright line test of fairness
The issue
72. I turn now to Issue I. In Phenytoin the Court (paragraph [97(i)]) held that “…in broad terms”, a price was unfair and an abuse when the dominant undertaking reaped trading benefits which it could not have obtained in conditions of ‘…normal and sufficiently effective competition’, i.e. ‘workable’ competition”. The applicants argue that the acid test is therefore whether the disputed prices are generated in a market characterised by a structure reflecting workable competition. If they are then they must be lawful even if they are way above Cost Plus. The applicants argue that the Cost Plus approach adopted by the CAT was wrongly based upon the persistence of market power, a failure to determine fairness by reference to pricing which would stimulate competition, and was divorced from any test of workable competition.
73. To support the centrality of market structure to the analysis the applicants refer to National Grid v Gas and Electricity Markets Authority [2010] EWCA Civ 114 at paragraph [85] where this Court noted that “competition rules promote consumer welfare indirectly by their effect on market structure and the promotion of competition”. Further, Case C-95/04 P, British Airways v. EU Commission ECLI:EU:C:2007:166 is cited where Advocate General Kokott stated:
“68. The starting-point here must be the protective purpose of Article [102]. The provision forms part of a system designed to protect competition within the internal market from distortions […]. Accordingly, Article [102], like the other competition rules of the Treaty, is not designed only or primarily to protect the immediate interests of individual competitors or consumers, but to protect the structure of the market and thus competition as such (as an institution) […] In this way, consumers are also indirectly protected. Because where competition as such is damaged, disadvantages for consumers are also to be feared.”
74. A sharper test for demonstrating workable competition was put forward focusing upon prices generated in a market where three cumulative conditions prevailed: (i) no market dominance; (ii) no collusion; and (iii); no insurmountable barriers to entry. Any price generated in such a market was necessarily lawful. Cinven produced for the Court a graphic which encapsulated its proposition:
Analysis
75. I do not accept the argument. There are four points to make.
The role of workable competition as a test for fairness
76. First, the graphic suggests that any price which falls below the horizontal line representing the threshold for workable competition is automatically lawful and they rely upon this to undermine the reliance by the CAT upon Cost Plus . In this the applicants wrongly elevate broad economic generalisations about workable competition into mandatory hard and fast principles of law. It is important to be clear about the implications of different phrases which are used in the case law. The term “workable competition” is a short hand for the language used in United Brands (ibid) paragraph [249] of “normal and sufficiently effective competition” and this, itself, is a shorthand for fairness, which is the legislative test. The concept of “workable competition” was formulated by John Maurice Clark in the American Economic Review in June 1940 in an article entitled “Towards a concept of workable competition” and was developed as an antidote to the economic concept of “perfect competition” which emerged in the late 19th century literature in which paradigm markets were described as in optimal equilibrium where output was equal to marginal cost. It was early understood, however, that the use of perfect competition as a tool for understanding how markets really operated, or for determining regulatory policy, was unrealistic and attention turned to workable competition as a practical alternative. This spawned an enormous body of literature over the decades. For example, Jesse Markham in June 1950, in a classic article entitled “An Alternative Approach to the Concept of Workable Competition” , highlighted both the utility of the concept but also its shortcomings including its lack of “precise definition”. Even today there is no consensus as to the exact parameters of the workably competitive market.
77. This judgment is not the place to embark upon an analysis of the literature. I observe only that there is agreement that competition law regulation does not proceed upon some theoretical, laboratory, model of perfect competition but upon the real world and focuses upon achieving the acceptable or adequate as opposed to the paradigmatic. Evidence of how a market reflecting “normal and sufficiently effective competition” or “workable competition” operates might therefore be relevant, and even important, evidence in a case but it is not a mandatory test. There is no rule that a regulator or Court must seek out evidence of what might happen in an actual market said to exhibit the features of workable competition as a benchmark. The premise which underlies the applicant’s graphic depiction of a bright line test is thus unsupported in the jurisprudence. The case law, as summarised in Phenytoin at paragraph [97] (see paragraph [24] above), describes practical approaches to determining fairness as the legislative test. It is understood that, to make the law practicable, there must be evidential proxies for determining what a fair price would be if generated in sufficiently effective, workably competitive, market conditions. It also makes clear that there is a wide range of economic and accounting models, as well as a variety of sources of evidence (e.g. comparables), that can be used to this end. As observed this does not mean that evidence of a broad nature about market structure is irrelevant but it does mean, contrary the applicants submissions, that in an appropriate case Cost Plus, is a valid and sufficient way of establishing whether prices are “fair” and, to this extent, can be said to reflect those that would be generated in a sufficiently effective, workably competitive, market: United Brands paragraphs [248]-[252] and Phenytoin paragraph [97(i)-(v)]. This is notwithstanding that a Cost Plus exercise is performed in relation to a dominant undertaking operating in a market which is not workably competitive.
The cessation of dominance: The persistence of abusive effects (contamination and price stickiness)
78. Secondly, at the most basic level it is correct that a market will not be workably competitive where there is a single monopolist or incumbent dominant undertaking. This is why the law imposes a “special responsibility” upon the dominant undertaking to act fairly. The market cannot bring about that result by itself, so the law plugs the gap. Accordingly, the cessation of that position of market power may be a factor relevant to a conclusion that a market is workably competitive. However, that analysis is incomplete.
79. The CMA and CAT both found, as a fact, that prices, post entry, were “contaminated” i.e. bore the lingering effects of the prior abuse. In other words the prior abuse by Advanz led, causally, to prices across the entire market (whether charged by the applicants or third parties) being higher than they should have been, upon a persistent basis. The continuing contamination was made possible by the uncompetitive nature of the market, even following new entry. In this respect given that entry occurred when the ASP was £247 (which no one has sought to defend economically as non-abusive ) compared to an average Cost Plus of £4.94, which the CMA and CAT found was reflective of prices in a competitive generics market, prices would have to tumble a long way before they reached some workably competitive equilibrium at which point in time it might be said that the effect of the abuse had disappeared from the system. The position of the CMA, which the CAT endorsed, was that a competitive market was in the proximity of Cost Plus.
80. In fact before the CAT there was no real dispute (Judgment paragraph [243]) that, even when dominance was lost, the rate at which prices would adjust from the towering height of £247 to normal competitive conditions would, to use the terminology of the experts and counsel, be “sticky” or “gelatinous”. Mr O’Donoghue KC, for Cinven, accepted that there would be some period of adjustment and that the rate of decline might be retarded (i.e. sticky or gelatinous). This was also the view of all the experts before the CAT. There was disagreement on the evidence as to whether prices had in fact fallen to competitive levels but there was a broad consensus that a period of adjustment would occur and that it would not be immediate.
81. In the Joint Experts Report the experts were asked to comment on the following proposition (at A.22):
“The evolution of prices for Liothyronine Tablets indicates that prices do not adjust immediately to competition. Instead, they show a degree of “stickiness”.”
Professor Valletti for the CMA replied: “Agree. [Valletti ¶68] This point is considered in the Decision at paragraph 5.311. Recent price data submitted by the CMA to the Tribunal confirm the aspect of “stickiness” in this market: prices keep decreasing continuously by about 30% a year and are expected to decrease further.” Dr Bennett for Cinven agreed that prices might yet fall to lower levels, but he thought that competition might still be workable. He replied: “Agree with a qualification: Liothyronine prices have adjusted over time (as opposed to quickly reaching a single price). However, I do not agree that the fact that prices have not reached their minimum level can be viewed as evidence that there is not workable or effective competition. [Bennett 1 ¶79].” Ms Jackson for Hg did not express a view as to whether prices had in fact fallen to their nadir, but she agreed that they do not stabilise at a lower rate immediately upon entry. She replied: “Agree. The CMA uses the term “sticky” to refer to prices that do not stabilise immediately at a new level post-entry, and I agree that this is the case in Liothyronine. However, I disagree with the CMA’s conclusion that this is evidence that competition in Liothyronine is insufficiently effective, and/or that post-entry prices 3.5 years or even nearly 5 years after entry are uninformative competitive benchmarks. [Jackson 4 ¶64-71, Jackson 6 §2]”.
82. The starting position adopted by the experts was plainly correct. References to workable competition in case law are used in a highly relative way. As the CAT correctly observed the test is not the mere existence of some competition. It is whether the competition that actually exists is “normal” or “sufficiently effective”, critical qualifications governing what is “workable”. The emergence of the first green shoots of competition in a market is not therefore an indication that the market is at that point necessarily “normal” or “sufficiently effective” or “workable”. That market might (but will not necessarily) arrive at a mature, workably competitive, state at some point in the future. In this context a test of contamination has to be correct. Otherwise, a sufficiently effective, workably competitive, market would be deemed in law to exist where there was enduring anticompetitive taint by prior abuse. The argument that post-entry the level at which prices settle is decisive evidence of a competitive market but which ignores the possibility that the settled price remains contaminated by the prior abuse, is not credible.
83. It follows that a bright line test which posits the cessation of dominance as a determinative condition, but which ignores the possibility that the post-dominance effects of abuse continue to distort a market, is incomplete and lacks evidential and economic support.
84. As an aside I note that there is no finding in this case by either the CMA or the CAT as to when dominance ceased to exist. It appears to have been accepted that dominance was lost at some point following entry by Morningside and Teva in 2017 but there is no finding as to when this tipping point was reached. This was because the CMA adopted, for administrative prioritisation reasons, the date of 1st July 2017 (immediately prior to first entry) as the cut off point for the formal finding of infringement and the imposition of penalties but has left open the question of breach thereafter: see paragraphs [43]-[47] above. It thus remains possible that Advanz was dominant for some period of time after the end of the Infringement Period.
The absence of collusion: The existence of conscious parallelism
85. Thirdly, the applicant’s test also assumes that absent collusion there can never be anti-competitive market effects which are equivalent to the distorting effects of collusion. This ignores the well-established possibility that markets where there is no dominance and no collusion can still be seriously uncompetitive because of what is sometimes termed “tacit collusion”, “conscious parallelism”, or “oligopoly pricing”.
86. This is an observable feature of markets typically characterised by: high barriers to entry; a relatively homogeneous product such that consumers can easily switch between competing suppliers; a small number of suppliers; and a degree of price transparency which means that competitors can monitor each other’s pricing policies. Pricing in such markets can reflect that occurring where there is collusion but without there being any actual inter-entity conspiracy. In such markets participants learn from observing the pricing behaviour of rivals that there is little to be gained from price competition. Over time competitors appreciate that they can maintain prices or even push prices upwards in the knowledge that the rational response from rivals is to follow suit and not seek to compete. They learn that the consequence of competing on price is that they retain the same volume of business but now at lower margins. In such a market, prices tend to stabilise at supra-competitive prices and can even rise, just as they might in the case of a covert cartel.
87. Legislation has long existed (such as the Fair Trading Act 1973 and the Enterprise Act 2002) creating powers of intervention to address such problems. In argument counsel for Cinven suggested that the reference to “collusion” in the graphic should be read to include an absence of tacit collusion and it was said that there was no evidence of such in the present case. I disagree. The CAT Judgment did not expressly use the expression tacit collusion (or “conscious parallelism” or “oligopoly pricing”) when concluding that, following new entry in 2017, prices remained uncompetitive and contaminated. But it accepted the analysis of the CMA that the market exhibited all the features of “soft” competition: three suppliers; protected by high barriers to entry; selling an essentially homogeneous product; to a fixed customer base that was unresponsive to price reductions; in relation to a product where there was a fair degree of price transparency; and where prices remained substantially above Cost Plus and relevant comparators. These are classic hallmarks of oligopoly: See for a more detailed analysis of the facts supporting this conclusion paragraphs [132]-[139] below.
The absence of insurmountable barriers to entry: The pro-competitive nature of high prices.
88. The applicants next contend that absent insurmountable barriers to entry high prices can be pro-competitive because they send enticing signals which stimulate competitive entry. In submissions paragraph [104] of Phenytoin was cited for the proposition that markets were self-correcting and in the absence of insurmountable barriers to entry regulatory intervention risked perpetuating monopoly by blocking efficient signals which would otherwise promote entry and new competition to undermine the incumbent monopolist. A belief in the self-rectifying properties of market forces was important and bolstered by a wide recognition of the high risk of regulatory failure.
89. The judgment in Phenytoin in fact made the opposite point. The Court in paragraph [104] did not support a test based upon the virtues of price signalling or express faith in the self-rectifying nature of markets. Paragraphs [103]-[105] contain a summary of economic literature relating to drug pricing which had been placed before the Court and they need to be read as a whole. They do not purport to lay down principles of law, as opposed to economic generalisations and observations which bear upon the way in which regulators internationally exercise their supervisory powers. Paragraph [103] in fact emphasised the importance of ex post price regulation even following patent expiry and specifically referred to the scenario, which aptly describes the present case, of third party investors identifying niche products the prices of which could be increased due to ineffective market and other regulatory constraints. In paragraph [104] the Court went on to distinguish such cases from those where there were “no material barriers” to entry where high prices might provoke new entry and healthy price competition. In paragraph [105] the Court explained that there was a wide variety of different categories of evidence identified in literature that could be a relevant to determining excessive and unfair pricing. Paragraphs [103]-[105] stated:
“103. [Counsel] drew our attention to various features of pharmaceutical markets recognised in the literature, to support the submissions of the CMA favouring a wide margin of appreciation for competition authorities and the importance of ex post intervention. The OECD Paper explains why such ex post intervention is relatively unusual but also how it can be very important in certain types of case. Medicines are subject to a "dense and comprehensive" regulatory framework that recognises the limited ability of competition enforcement agencies to lower prices. The framework is less comprehensive in relation to off-patent drugs, where inter-brand competition is relied upon to contain prices. But competition concerns can arise even in the off-patent sector where there can remain an absence of therapeutic and inter-brand competition even upon expiry of patents. This can lead to a "lack of price elasticity of demand, particularly as regards 'essential' drugs". These developments appear, observationally, to have occurred in tandem with the emergence of business strategies that identified market segments where prices could be successfully increased. Companies identify niche essential drugs that are not under patent but whose market is so small that no competitors will enter the market, or where supply is limited for regulatory or contractual reasons. In its submissions the CMA identified analogous factors specific to the drug in question in these proceedings which it contended made the present case apt for ex post intervention. These included that phenytoin was not in patent. It is an "old" product first marketed in 1938 which was used for a declining patient population but where the suppliers benefited from regulatory clinical guidance which substantially precluded switching even between clinically identical, molecular, substitutes. This served to maintain barriers to switching and inter-brand competition and was an important factor in establishing the dominant positions of Pfizer and Flynn in their respective markets and their power over price.
104. These features served to distinguish the present case from other markets where patent expiry removed the principal obstacle to market entry. Where there are no material barriers to entry high prices can act as a magnet to entry which, in due course, drives prices down. Many markets are thus self-correcting. In the absence of entry barriers regulatory intervention can risk prolonging a monopoly situation by blocking efficient signals which would otherwise promote market entry. A belief in market forces "…is often bolstered by the (perceived high) likelihood of regulatory failure, a risk which is compounded in the case of price regulation". The investigation of ex post cases of alleged excessive pricing faces significant difficulties in terms of data availability and analysis, identifying appropriate assessment standards, and of designing and implementing suitable remedies: "This has led some to consider that the identification of excessive prices is a 'daunting, if not, impossible task'… The issues are still more extreme when trying to set clear rules that allow for ex ante compliance with excessive pricing rules. The key problem here is that it is not clear what the appropriate benchmark should be.". In written submissions the CMA argued that the economic difficulties in applying competition law to excessive pricing must not be allowed to render "…the law a dead letter". It was the task of the competition authority to exercise its judgment as to when ex post intervention was apt, and courts should avoid articulating rules which made this inherently difficult task unworkable or excessively difficult.
105. The pharmaceutical companies cited the OECD Paper because it described the many different approaches which authorities use to evaluate whether prices are exploitative/unfair. The OECD Paper cites United Brands as laying down the seminal test in the EU and observes that European competition authorities and courts have made use of a variety of different methods, all said to be consistent with the case law, to determine whether a price is excessive and unfair. In some cases, a comparison between production costs and prices is used but price/cost analysis is not feasible in all cases due to lack of data or because the disputed price relates to an intangible good such as an IP right. Other methods are also used such as benchmarking "of some sort". Price-based benchmarks are used by comparing the investigated price with prices charged by the dominant firm in different markets or over time or by comparing the prices charged by the dominant firm and those charged by other firms, either in the same market or in other markets. Another benchmark focuses upon the profitability of the dominant firm by comparing such profits either with a normal competitive profit or the profits of other firms. Other methods are also identified. The guiding factor in each case is the availability and suitability of the evidence and data. Competition authorities often adopt a pick and mix or combinatorial approach to the evidence to be relied upon. There are no fixed rules, assumptions or presumptions. Everything depends upon the facts of the case.”
90. The argument that the Court in Phenytoin endorsed a structural test embracing the absence of “insurmountable” barriers to entry is thus incorrect. In the present case the CAT found that the barriers were not insurmountable but nonetheless remained “exceptionally” high (Judgment paragraph [318]). In relation to generalisations about the positive effect of prices which encourage entry, this was limited to markets where barriers to entry were not material, i.e. were low. Even as a (non-legal) generalisation this is the opposite end of the spectrum from a structural condition precedent for workable competition proposed by the applicants based upon the absence of “insurmountable” entry barriers.
Conclusion
91. On the facts of the case, as found by the CMA and the CAT, the market following new entry exhibited a range of features strongly redolent of ineffective competition which enabled the effects of the prior abuse to endure, even following the cessation of dominance. This was the answer of the CAT to the applicant’s case that observable prices were properly explicable (only) by virtue of the operation of a workably competitive market.
92. Pulling threads together there are two points to make. First, any iteration of structural market conditions said to amount to a bare minimum for workable competition would need to include: (i) a proper adjustment period once dominance was lost for any persistent contaminating effects of the abuse to come to an end; and (ii) an absence of any evidence or risk of ineffectively competitive market conditions which allowed the effects of prior abuse to endure; and (iii), sufficiently low barriers to entry. The second point is that whilst market structure may play a part in the analysis, in law it amounts only to evidence (albeit potentially important evidence) relevant to the question whether the competition that now exists is “sufficiently effective”. It is not a mechanistically dispositive test for the fairness and hence legality of pricing.
H. Issue II: The relevance to fairness of Entry Incentivising Prices (“EIP”)
The issue
93. The second issue is different to the first. It is argued that the market price at which the entry process was incentivised (the EIP) evidences workable competition and, as such, reflected prices that are “fair”. This differs from a price arising under Issue I because a price which incentivises entry may occur before or after the concurrence in time of the structural conditions said be relevant under that Issue. To be specific it is not said that the market price which prevailed at the point in time of actual entry is relevant (actual entry occurred when the price was c.£247 in summer/autumn 2017). The EIP is the prevailing price which a putative entrant considered, internally, that entry was viable. This might be some years before actual entry. Before the CAT evidence was adduced as to the prices which, it was said, incentivised third party entry. The applicants did not pick upon a single price but put forward a range. For instance, the expert for Hg (Ms Jackson) adduced evidence that the EIP was in the region of £45-£95 (see Judgment paragraph [301]). The applicants also argued that because Advanz knew that by pushing prices up, new entry would likely be stimulated and new competition facilitated this was evidence of a pro-competitive intent and militated decisively against a finding of abusive unfairness.
94. The CAT rejected the submission that an EIP was a benchmark test consistent with general principles of competition law but it accepted that it could form a part of the relevant evidential mix. It also rejected the submission that knowledge or foresight that high prices would stimulate entry was relevant. It did find that if EIP were relevant “a” price that reflected a viable entry point was £21, which was much lower than the price evidence adduced by the applicants.
95. The issues before this Court are whether the CAT erred when it: (i) concluded that in principle this sort of evidence was not determinative of fairness; (ii) rejected the evidence of the applicants; and (iii), concluded that if EIP were relevant then a much lower price (i.e. £21) than that posited by the applicants represented a viable EIP.
The CMA Decision
96. It is informative to start with the reasoning in the Decision which the CAT endorsed. The CMA rejected EIP as a test : See Decision paragraphs [5.347]-[5.354]. In summary:
(i) It would undermine the effectiveness of the statutory prohibition. If the CMA could not intervene against prices below an EIP an incumbent could extract very high profits from consumers indefinitely by pricing at a level many multiples above Cost Plus but slightly below the EIP (“limit pricing”). It would have the perverse effect that the higher the barriers to entry protecting the incumbent, the higher the profits it could extract by limit pricing: Decision paragraph [5.349].
(ii) The argument that it was important in law that Advanz knew that its pricing strategy would stimulate entry which would necessarily lead to competition and a fall in prices to a competitive level was irrelevant and wrong. In a genuinely “effectively competitive market” a temporary period of higher prices which led promptly to entry and a return to effectively competitive pricing over a reasonable time period might not result in material consumer harm. But where barriers to entry were high the converse could apply and market forces could not be relied upon to self-correct within a reasonable period. In this case nine years after commencement of the first successful entry attempt prices remained very high. The price of the tablets was £45.52 per pack in September 2012, when Morningside began its entry attempt and £65.64 nine years later in February 2021. Entry had failed to result in effective competition: Decision paragraph [5.350]. The intention of Advanz throughout was to exploit market inefficiency to maximise profits regardless of the prospect of new entry. There was no evidence suggesting that it acted to bring about a competitive market.
(iii) The emergence of new competition was in any event not a guiding objective of competition law. Had Advanz exercised price restraint and priced at or proximate to Cost Plus (irrespective of whether that led to new entry) consumers would have benefited much more than under a test based upon EIP where, as the present case demonstrated, prices were now many multiples of Cost Plus. The NHS would have been significantly better off if Advanz had not de-branded and had continued to be subject to the PPRS, even if this would have resulted in entry being “precluded indefinitely”. In such a case the NHS would have been significantly better off: Decision paragraphs [5.352] and [5.354].
(iv) New entry had resulted in no increase in quality or innovation generating material efficiencies to offset the higher cost to the NHS. Liothyronine Tablets were an old, long established, drug with inelastic demand and limited scope for improvement. New entry had not generated the sorts of non-price benefits that competition could be expected to stimulate such as: increased output, quality improvements, the enhancement of efficiency, or the introduction of new and better products, etc: Decision paragraph [5.353(b)].
Conclusions of the CAT
97. The CAT endorsed the conclusion of the CMA: Judgment paragraphs [317]-[325]. Its reasoning can be summarised as follows.
98. First, EIP were not a valid, dispositive, test (Judgment paragraphs [318] and [319). The law existed to protect consumers from unfair treatment when a dominant undertaking was freed from competitive shackles (see e.g. London & South Eastern Railway Ltd and others v Gutmann [2022] EWCA 1077 at paragraphs [93]-[102]). The level of EIP in a market with high barriers to entry had to be sufficiently high to give third parties an incentive to surmount the barriers and it followed that the higher the barrier to entry, the higher the EIP. In the present case barriers to entry, though not insurmountable, were “exceptionally high”. Therefore, a price charged by an incumbent dominant supplier could be excessive and unfair even below the level required to incentivise entry. Treating EIP as a benchmark allowed a dominant undertaking to exploit high entry barriers to the detriment of consumers. The argument that the CMA could not set prices below viable EIP levels was flawed because in a market with exceptionally high entry barriers there might never be entry. The law did not identify any such jurisdictional bar. The economic literature cited by the applicants did not support the argument that price regulation was justified only where barriers to entry were insurmountable but, instead, assumed that there would be no material barriers to entry. For these reasons, treating EIP’s as dispositive of fairness was inconsistent with settled case law. The CAT observed (Judgment paragraph [319]):
“… it would give primacy to the furtherance of competition, regardless of whether the trading benefits reaped by the dominant undertaking from charging Entry-Incentivising Prices were vastly higher than the prices achievable in normal and sufficiently effective competition and regardless of whether there was a reasonable relationship between the Entry-Incentivising Price and the economic value of the product in question.” … .
99. Secondly, the argument was not economically viable. It was common ground between the experts that EIP did “… not reflect the outcome of an effectively competitive process…” but represented only “… the beginnings of the competitive process”: Judgment paragraph [317].
100. Thirdly, use of an EIP was an inherently unreliable benchmark because that which incentivised entry was determined by “… subjective intentions, circumstances and actions of third party entrance, their access to capital and their approach to risk and on the pricing conduct of the dominant undertaking” (Judgment paragraph [320]). An EIP could not therefore be treated as a “proxy for a workable competitive price as the dividing line between a fair and unfair price”. It could not be a “hard and fast benchmark”.
101. Fourthly, as to the complaint that Cost Plus precluded entry and thereby the benefits which could flow from such entry, it was no part of the test in law that the price benchmark for fairness had to be set at a level which facilitated new competition in order to create a possibility for product improvement:
“321. … The test does not presuppose that the potential benefits of competition are such as to justify and render non-abusive whatever price is needed to incentivise other entrants to compete. Nor does the test require a comparison to be made between, on the one hand, the benefits of competition with, on the other hand, the harm resulting from excessive prices. If such a comparison were to be made, the CMA concluded in the Decision that the incremental improvements which have been made in the provision of Liothyronine Tablets since Teva and Morningside began to compete (assuming in the Appellants’ favour that these improvements would not have occurred had Advanz remained as sole supplier of Liothyronine Tablets), that is to say the availability of different dosages, a longer shelf life, a lactose free option and increased security of supply - were disproportionately small to justify the increase in prices needed to stimulate entry. We agree with that assessment. Liothyronine Tablets are an old and established drug with limited scope for improvement.”
102. Fifthly, in relation to the argument that high prices which acted as a magnet to new entry should be treated as intrinsically “fair”, because they were implemented in the “knowledge” that they would eventually stimulate entry and price competition, the CAT (Judgment paragraph [322]) cited this Court in Phenytoin which made clear that if there was any mileage in the argument it could only ever be in a market with low entry barriers:
“As to the further argument that Advanz’s price increases were not unfair because Advanz implemented them in the knowledge that they would lead to new entry, increased competition, and a subsequent reduction in prices, this argument would have validity in an effectively competitive market as mentioned … in Phenytoin:
“Where there are no material barriers to entry, high prices can act as a magnet to entry which, in due course, drives prices down. Many markets are thus self-correcting.””
Where there were high barriers to entry, an intention to push prices up to or beyond the EIP could not be said to reflect a pro-competitive intention. Self-correction would not necessarily occur within a reasonable time.
103. Finally, were EIP to amount to a useful benchmark the CAT would have taken the price of £21 as an EIP being the prevailing price in 2010 when Uni-Pharma commenced its (ultimately unsuccessful) attempt to enter the market. The CAT observed that on the evidence that price appears “… to have been considered by Uni-Pharma to be a viable price which merited significant work and costs” (Judgment paragraph [325]).
Applicants’ submission
104. The applicants put forward 5 main points before this Court.
105. First, the relevance of EIP was established by the CAT in Napp Pharmaceutical v Director General of Fair Trading [2002] CAT 1 (“Napp”) which endorsed the observation in the decision under appeal in that case which said (paragraph [203]) that, in principle, a price was excessive:
"…if it is above that which would exist in a competitive market and where it is clear that high profits will not stimulate successful new entry within a reasonable period. Therefore, to show that prices are excessive, it must be demonstrated that (i) prices are higher than would be expected in a competitive market, and (ii) there is no effective competitive pressure to bring them down to competitive levels, nor is there likely to be."
Where entry occurred, there was “… effective competitive pressure to bring them down to competitive levels…”. Accordingly, EIP were a valid threshold test for fairness and were consistent with competition law policy which favoured the creation of competitive market structures and which undermined incumbent (dominant) market power. The CAT erred in finding to the contrary.
106. Secondly, the CAT erred in failing to attribute legal and evidential weight to the unequivocal fact that Advanz priced (upwards) knowing that this would stimulate new entry. This applied even in relation to a situation of high entry barriers. Entry attempts were thus stimulated by Advanz’s pricing behaviour, which increased the market size (by value of sales, though not volume) with the result that entry became increasingly attractive in terms of the potential revenues new entrants could earn. Prices were fair because they were charged knowing that they would lead to new entry, increased competition and a fall in prices.
107. Thirdly, the evidence was inconsistent with the price of £21 found by the CAT to be a viable entry price from the perspective of Uni-Pharma (see paragraphs [48]- [50] above). An EIP in the range £45-£95 was the relevant benchmark. A price of £62 was the average charged by the new entrant Teva across the period 2013/2014 which was conservative given: (i) that (as recognised in the Decision paragraph [4.142]) the actual price which incentivised entry by Teva was £94.63; and (ii), in closing submissions to the CAT Advanz explained that the EIP was in a range of £60/£80. The lowest credible EIP was £45.52 which was mentioned in the Decision at paragraph [5.347] as reflecting the point when Morningside began its attempts to enter. In choosing £21 the CAT was internally inconsistent and wrong.
108. Fourthly, the approach of the CAT was contrary to the statutory duty on the CMA to “promote competition” for the benefit of consumers under Section 25(3) of the Enterprise and Regulatory Reform Act 2013. A price above the EIP was a signal which promoted competition consistent with the law and was therefore “fair” and lawful. A price capped at Cost Plus would never induce entry and would never stimulate competition. The Cost Plus model required dominant undertakings to price at a level which foreclosed entry even though the “special responsibility” imposed upon a dominant undertaking was not to act in a way that foreclosed competition which, when allowed to operate, brought about innovation and security of supply. Absent insurmountable entry barriers, the CMA lacked jurisdiction to intervene against prices below the EIP. Cost Plus was invalid as a benchmark for pricing in a workably competitive market because it was based upon the costs of a monopolist, and not those pertaining in a competitive market.
109. Finally, the CAT wrongly ignored evidence of the benefits which had in fact been generated by new entry. They were causally connected to the high prices and the CAT was required to take them into account as part of the justification for the prices themselves.
Analysis
110. I do not accept that the CAT erred. The reasoning of the CAT in endorsing the analysis of the CMA, summarised above, is sound. I start with conclusions relating to the evidence and then turn to the law.
Conclusions on the evidence
111. First, the case of the applicants assumes that there is a nexus between the point in time at which an undertaking first contemplates entry and the effectiveness of competition in the market. As a matter of basic logic and economics there is no such link:
(i) A market which is uncompetitive and likely to sustain higher prices is, commercially, more attractive as one to invest in than one characterised by vigorous competition which will push prices closer to Cost Plus. The suggestion that an EIP reflects a price generated in a market that is considered by the putative entrant to be workably competitive is also belied by the evidence. The internal documentation relating to the periods of ownership of Hg, Cinven and Advanz Pharma Corp strikingly demonstrates that the parties were attracted to the market for the very reason that it was not seen as competitive in any sense, workable or otherwise, and this incudes for instance Cinven when it acquired the business from Hg: See the summary at paragraphs [59]–[70] above. There is no basis for distinguishing between the motives of the Advanz entities and third parties (such as Morningside or Teva) who entered subsequently to compete with Advanz and were predicting prices in a market which had shifted from monopoly to duopoly and then to oligopoly. True it is that the numbers of suppliers changed, but the fundamentals of market structure which facilitated supra-competitive pricing did not (see paragraphs [132]-[139] below). A potential new entrant might commence the work necessary to enter but make the final entry decision contingent upon the trajectory in price. Entry might be delayed in the expectation that the incumbent would drive prices up knowing that once entry has occurred and price competition has been triggered, since prices started from a dizzy height, they would settle at higher ground than otherwise would have been the case had entry occurred earlier and had the market been genuinely competitive. There is no mystery about any of this. It is business common sense.
(ii) The case of the applicants illogically divorces the EIP from the price at the point of actual entry, which might be many years later. An EIP, as described, is a price artificially fixed by reference to a point in time which almost by definition is years in advance of the commercial pricing decisions the supplier will subsequently take when it actually enters the market. For instance, if the EIP is fixed at £65 but upon new entry, some years later, the price collapses to £10 in response to the emergence of some real competitive market forces, the dominant incumbent would still contend that £65 reflected a workably competitive market, even in the face of hard evidence as to what happened to prices when there was competition.
112. Secondly, the CAT was plainly correct as to the vagaries of EIP as a source of evidence. It will, invariably, be difficult to determine the exact price which stimulated entry (see paragraph [100] above). In addition, it will be confidential information not available to an incumbent dominant undertaking and comes up against issues of legal certainty: See paragraphs [142]-[145] and [202] below.
113. Thirdly, reflecting all of this, the consensus view of the experts was that EIPs were admissible as evidence but did not amount to a definitive test or benchmark for fairness. The experts joint report bears this out. They agreed with the proposition in C.1: “Entry-incentivising prices are not the outcome of an effectively competitive process”. They also, albeit with different emphases, agreed with the proposition at C.7 which concerned the probative value of EIPs:
“Conclusion: Entry-incentivising Prices are not an informative benchmark for effectively competitive prices for Liothyronine.”
Professor Valletti for the CMA replied: “Agree: in the presence of high fixed entry costs, entry-incentivising prices must necessarily be higher than affectively competitive prices. Moreover, accepting entry-incentivising prices as a relevant benchmark would amount to saying that an incumbent should be allowed to price higher, the higher the barriers to entry faced by potential competitors.” Professor Valletti did not squarely answer the question because he disagreed with the underlying premise that EIP could be a “benchmark” at all, and he did not therefore express a view on whether it could provide any relevant information. Dr Bennett for Cinven said: “disagree: Entry incentivizing prices are relevant especially when taken in the round with other evidence.” Ms Jackson for Hg said: “Disagree. Although not direct evidence of competitive price levels, Entry-incentivising prices are still of interest (alongside other competitive benchmarks) in seeking in assessing whether prices actually charged were excessive.” She reiterated the point in her answer to Proposition C.5 where she emphasised that she did not suggest that EIPS “should be used as a standalone benchmark”, only as one source of information amongst others.
114. Fourthly, the CAT held that assuming EIP to be relevant “a” price of £21 was treated by Uni-Pharma was viable: see paragraph [48]–[54] above. The challenge to this reflects a disagreement about a finding of fact over which the Court of Appeal has no jurisdiction. It is not credible to say that the CAT mischaracterised the evidence. It was entitled to conclude that Uni-Pharma considered this to be “a” viable entry point. It is not a valid objection to say that ultimately the company did not enter the market. That fact might be relevant but only as one piece of the evidential jigsaw making up a conclusion as to what, internally, a third party considered a viable entry price point to be.
115. Finally on the evidence, I turn to the issue concerning benefits introduced by new entrants. The argument is that these benefits would not have materialised but for new entry which itself would not have occurred but for very high prices. The benefits are therefore proof of the pro-competitive and pro-consumer nature of high EIP. It is not however said that Advanz knew about these benefits, welcomed them, or intended them as a consequence of its high pricing. In Annex V of its Closing Submissions to the CAT, Advanz described the benefits which it said flowed causally from the prices it charged which induced the entry which, in turn, brought about the benefits. These were summarised as follows:
“A new 5mcg and 10mcg Liothyronine tablet emerged, which meant that patients themselves no longer had to divide the larger 20 mcg Liothyronine tablet. This is important clinically because patients themselves were attempting to give themselves a smaller dosage in a very rudimentary way: for example, using a knife to divide the tablet, or crushing the tablet in powder and estimating the relevant grams. As Liothyronine is extremely potent these approaches were far from efficacious, as obviously there is considerable scope for error. Indeed, the regulator, the MHRA, expressly asked Morningside to manufacture the smaller 5mcg and 10mcg dosage tablets to meet a “clear clinical need”.
A tablet with a longer shelf-life of 24 months compared to Advanz’s tablet with a 12-month shelf life was introduced. As the Tribunal noted at [250]…pharmacies had specifically requested a Liothyronine tablet with a longer shelf-life. This is not simply about pharmacies’ commercial interests (e.g., the cost of re-ordering), but a longer shelf life extends the chemical stability of the active ingredient.
A lactose-free Liothyronine tablet was introduced, which obviously benefits lactose intolerant patients.
A capsule version was introduced. This is easier to swallow than the tablet which is important for more elderly patients.
The supply of Liothyronine itself was made more secure, as there were new sources of manufacture. Surety of supply is a critical policy consideration of the DoH as Advanz showed in Annex 5 to its Written Closing Submissions (and noted by the Tribunal at [250] … and [297] - [298]…). The evidence before the Tribunal was that Advanz had experienced repeated shortages in the supply of Liothyronine as it is difficult to manufacture. New market entrants reduced the probability and thus the risk of such shortages in supply occurring.”
116. The CAT rejected this argument both upon the evidence and in law. It agreed with the CMA that on the facts such benefits as had materialised were disproportionately small and did not justify the price increases. It held that the law did not “… require a comparison to be made between, on the one hand, the benefits of competition with, on the other hand, the harm resulting from excessive prices”: See Judgment paragraph [321].
117. I have reviewed the evidence of benefits before the CAT. It is decidedly modest. In argument attention was focused upon the innovation brought about by the introduction of a new 5mcg and 10mcg Liothyronine tablet which meant that patients who benefited from lower dosages did not have to break up the larger 20 mcg Liothyronine tablet into smaller pieces. Of all the proffered benefits this seems the most substantial. The Court was shown data which revealed however that during the period prior to new entry there had in fact been a 5mcg and 10mcg option available on the market. This had been supplied by means of importation under the applicable import licence regime. The introduction of the lower dosages did not add anything new to the market. It created a domestic source of supply alongside the import. There was, significantly: no material evidence put before the CAT that any patient who benefited from the smaller dosages had been deprived of it prior to the new entry; no evidence establishing how or why having a domestic supplier generated material advantages relative to the import; and, no exercise undertaken to quantify the benefits and correlate them, causally, to the headroom between Cost Plus/£20.48, and the ASP. There was a hint that there were security of supply benefits from having additional sources of supply and that the MHRA welcomed the development but, with respect, the evidence on this was thin and unsubstantiated.
118. In my judgment if the applicants wished to argue that the supposed benefits were material it was for them to adduce detailed evidence of the magnitude of the benefit and its ability to offset increases in price. They were in a superior position (relative to the CMA) to collate and tender this evidence. In Case C-307/18 Generics (UK) Ltd and Others v CMA ECLI:EU:C:2020:52 (“Generics (UK)”) the CJEU confirmed the existence of a fairly rigorous evidential burden upon the dominant undertaking seeking to justify conduct:
“165 That said, it must be recalled, in response to Question 10(b) and (c), that, in accordance with settled case-law, it is open to a dominant undertaking to provide justification for behaviour that is liable to be caught by the prohibition under Article 102 TFEU, in particular by establishing that the exclusionary effect produced by its conduct may be counterbalanced, or outweighed, by advantages in terms of efficiency that also benefit consumers (see, to that effect, judgment of 27 March 2012, Post Danmark, C‑209/10, EU:C:2012:172, paragraphs 40 and 41 and the case-law cited).
166 To that effect, it is for the dominant undertaking to show that the efficiency gains likely to result from the conduct under consideration offset any likely negative effects on competition and the interests of consumers in the affected markets; that those gains have been, or are likely to be, brought about as a result of that conduct; that such conduct is necessary for the achievement of those efficiency gains, and that it does not eliminate effective competition, by removing all or most existing sources of actual or potential competition (judgment of 27 March 2012, Post Danmark, C‑209/10, EU:C:2012:172, paragraph 42), and consequently that undertaking has to do more than put forward vague, general and theoretical arguments on that point or rely exclusively on its own commercial interests.”
Similar positions relating to the evidential burden upon investigated or defendant undertakings have been expressed in Phenytoin at paragraphs [114] and [116], and in Sainsbury’s Supermarkets Limited v Visa Europe Services Limited [2020] UKSC 24 at paragraph [216]. In the internal documents disclosed to the CMA and the CAT, Advanz was of the clear view that there was little if any scope for product development and innovation (see paragraphs [66] and [68] above). On the basis of such evidence as was tendered the CAT, endorsing the conclusion of the CMA in the Decision, was entitled to conclude that the “benefits” were disproportionately small. This was a finding of fact, not law.
Conclusions on the law
119. I turn from the evidence to conclusions on the law. The points of law raised must be placed into the context of the fact that the experts were of the consensus opinion that EIP did not amount to a test but were simply part of the evidence mix. I will nonetheless take the arguments at face value and address them. The issues raised concern: (i) whether the judgment of the CAT in Napp (ibid) mandated EIP as a test of fairness and whether the CAT in the present case wrongly dismissed EIP as irrelevant in all cases; (ii) the extent to which the law endorses EIP as a test because it undermines dominance and leads to a more competitive market structure; (iii) the implication of the fact that on the applicant’s case limit pricing is acceptable; (iv) the relevance of Advanz’s belief that high prices would induce entry; and (v), whether an evaluation of benefits as a justification for otherwise abusively high prices is mandatory in law.
120. First, contrary the applicants’ argument, the judgment in Napp does not establish EIP as a relevant test of fairness. That case did not concern EIP. The CAT in that case (paragraph [391]) said of paragraph [203] of the disputed decision (see paragraph [105] above) only that whilst there were other ways of determining fairness the: “… Director's starting point, as stated in paragraph 203 of the Decision, seems to us to be soundly based in the circumstances of the present case." (emphasis added). In Phenytoin, the CAT judgment in Napp was treated by the Court as an exemplar of the combinatorial approach to evidence (see Phenytoin paragraphs [92]-[94]). In paragraph [92] the Court summarised the categories of evidence relied upon in the case. There were six: none involved an analysis of EIP. Napp does not preclude the possible relevance of an EIP; but it does not mandate it, nor accord it any elevated evidential significance. Insofar as the applicants argue that because of the judgment in Napp EIP are dispositive in law of fairness, I conclude that such a proposition is not arguable. On the other hand in the present case, I do not read the Judgment as holding that EIP are, in principle, always irrelevant as part of the admissible evidence. The CAT did hold that EIP “as a valid benchmark” was wrong in principle (Judgment paragraphs [318]). But it also accepted that EIP amounted to evidence of “…the beginnings of an effective competitive process” (Judgment paragraph [317]) indicating that it had a role to play as part of the evidence. I read the reference to a “benchmark” as referring to EIP as a test of legality, as opposed to a piece of evidence to be weighed in the balance. This is consistent with the case law. It is not hard to see how evidence of EIP might be informative. If the EIP had been, say, in the range £6.00-£8.00, which is relatively proximate to Cost Plus, it might have been a useful indication of the degree to which the market was genuinely competitive. However, on the facts, EIP advanced by the applicants were about ten times higher than 2007 prices, which were close to Cost Plus, and were more cogently explained by reference to the fact that the new entrants (Morningside and then Teva) identified the market as structurally uncompetitive such that even once the monopoly became a duopoly and shortly thereafter an oligopoly, all market players could still exploit the inefficient structure to reap very high, supra-competitive, profits.
121. Secondly, the applicants attach legal significance to the point at which new entry occurs upon the basis that dominance is a bad thing. The law should be directed at facilitating more competitive market structures which encourage new entry to dissipate incumbent market power. The premise that the law is directed at undermining dominant market power is however wrong. It is trite that dominance, per se, is not unacceptable. It is its abuse which is proscribed. Dominance can be the legitimate reward for innovation and creativity. Where dominance exists the law imposes a “special duty” not to misuse the attendant market power. If the dominant undertaking adheres to this duty, for instance, by not pushing prices up unfairly to the detriment of consumers, it is irrelevant that dominance persists, and new entry is not facilitated. There is no principle of law that prices should be set so as to facilitate the loss of dominance by encouraging new entry, and the judgment in Phenytoin does not lay down such a proposition.
122. Thirdly, as to “limit” pricing, the applicant’s argument is that it is only prices above the EIP which are abusive. On this premise a dominant undertaking can lawfully set a limit price just below the EIP which will still deter entry, even though that will still reflect a substantial increase over Cost Plus and bear no relation to comparables. The argument is that the steeper and more arduous the barriers to entry, the higher the level a dominant undertaking may price to, and the higher the (lawful) limit price, even if it involves an exorbitant return. I agree with the CMA and the CAT that this simply does not reflect the law and it has not been endorsed in a judgment. The prohibition upon abuse of a dominant position by unfair pricing treats excessive pricing as an exploitative abuse because of its effect upon consumers. This is why a Cost Plus test, which permits regulators and courts to take account, in the “plus” or fairness components, of a range of factors which includes a reasonable rate of return and questions of “value”, is a test which strikes a balance between the commercial and the consumer interests.
123. Fourthly, there is the argument that EIP are fair because the applicants intended to price to, and above, that point knowing (or at least believing) that this would lead to entry and the generation of price competition with a consequential reduction in prices. I disagree. In law evidence of an undertaking’s subjective intent or strategy is not a necessary requirement for proof of an abuse, but it can be inculpatory where it exists. In (Generics (UK) Ltd) (ibid) the CJEU, on a reference from the CAT, addressed a series of questions on the definition of abuse and the extent to which it applied to agreements between an incumbent pharmaceutical company and a potential entrant designed to deter entry. The CJEU considered the issue of subjective intent:
“162. To that effect, it must also be recalled that, while, for the purposes of application of Article 102 TFEU, there is no requirement to establish that the dominant undertaking has an anticompetitive intent, evidence of such an intent, while it cannot be sufficient in itself, constitutes a fact that may be taken into account in order to determine that a dominant position has been abused (see, to that effect, judgment of 19 April 2012, Tomra Systems and Others v Commission, C‑549/10 P, EU:C:2012:221, paragraphs 20, 21 and 24).
163 In this case, the CMA and the referring court consider that the conclusion by GSK of the agreements at issue was part of an overall strategy pursued by GSK to maintain as long as possible its monopoly position in the United Kingdom paroxetine market.
164 Consequently, if those matters are established, any anticompetitive intent on the part of GSK must be taken into consideration by the referring court in order to assess whether the conduct of GSK must be characterised as ‘abuse of a dominant position’ within the meaning of Article 102 TFEU.”
Thus the test is primarily objective; but evidence of subjective or strategic intent can be taken into account as inculpatory, where it exists. A genuinely mistaken belief that abusive conduct was pro-competitive could never therefore be exculpatory. On the facts as found, Advanz drove pricing upwards to optimise profits and did no more than acknowledge that at some point such a strategy would likely stimulate entry: See paragraph [67] above. This is neither philanthropic nor pro-competitive and the facts as found by the CAT in relation to the strategic intent of Advanz are capable of reinforcing the conclusion of the CMA and the CAT that its prices were abusive.
124. Finally, in respect of the law relating to benefits, the CAT rejected the applicants’ case on the evidence (e.g. Judgment paragraph [216]), so its position on the law is immaterial. Nonetheless, it did say (Judgment paragraph [321]) that the test did not require a comparison to be made between, on the one hand, the benefits of competition with, on the other hand, the harm resulting from excessive prices. But equally it also accepted that the existence of benefits could be a factor relevant to the assessment of the “plus” in Cost Plus or as part of an assessment of “value”: see Judgment paragraph [216]. The applicants cite the judgment of the CJEU in Case C-413/14 P Intel v Commission ECLI:EU:C:207:632 at paragraph [140] where the CJEU cited with approval Case C-95/04 P British Airways v Commission [2007] ECR I-02331 at paragraph [86]:
“Assessment of the economic justification for a system of discounts or bonuses established by an undertaking in a dominant position is to be made on the basis of the whole of the circumstances of the case (see, to that effect, Michelin, paragraph 73). It has to be determined whether the exclusionary effect arising from such a system, which is disadvantageous for competition, may be counterbalanced, or outweighed, by advantages in terms of efficiency which also benefit the consumer. If the exclusionary effect of that system bears no relation to advantages for the market and consumers, or if it goes beyond what is necessary in order to attain those advantages, that system must be regarded as an abuse.”
The CMA counters that neither authority indicates that any balancing of this nature was required in an exploitative pricing case; the judgments concerned allegations of abuse by pricing intended to be exclusionary of existing and future rivals, not exploitatively high prices directed at consumers, and they were thus distinguishable. Like the CAT, I would not go to the extreme of saying that an analysis of benefits is always irrelevant in an unfair pricing case in particular as part of the assessment of “value” or fairness under the Cost Plus test. Both Intel and British Airways are examples of the broader proposition that conduct that is prima facie abusive might be subject to objective justification. In Generics (UK) Ltd at paragraph [165] and [166] set out above (cf paragraph [118]) the CJEU was addressing the benefits flowing from an exclusionary abuse, but it started its analysis from the recognition that otherwise abusive conduct could be objectively justified and it proceeded to say that benefits might provide such justification. If the lynchpin for the analysis is objective justification, which can apply to all types of case, and benefits are an example of such a justification, then these judgments provide support for the proposition that the principle of justification (including by benefits) can extend beyond exclusionary conduct. I do however consider it to be an open question whether an otherwise exploitative and abusive price by a dominant undertaking could ever be justified upon the basis of extraneous benefits to consumers arising from the actions of third parties who enter in response to the abusively high price. I agree with the CMA that there is no authority supporting this particular proposition. It could well be inconsistent with the concept of dominant undertakings having a “special responsibility” to protect markets that they should be able, in an entirely serendipitous and opportunistic manner, to take advantage of market benefits for which they were not directly responsible and/or had no knowledge of and/or did not intend to come about. The CJEU in Generics (UK) Ltd at paragraphs [165] and [166] seems to suggest that benefits can be taken into account when they amount to “efficiency gains” attributable to the dominant undertaking itself; not to efficiency gains brought about by a third party for which the dominant undertaking can claim no credit. In British Airways (see paragraph [86] cited above) the Court held that where the (exclusionary) abuse bore “…no relation to advantages for the market and consumers…”, then the impugned conduct amounted to an abuse. There is thus a real question mark over whether the causal nexus or relationship that must exist between the erstwhile abuse and the benefit can extend to collateral efficiencies generated by third parties.
Overall conclusion
125. For all the above reasons I conclude that: (i) the challenge to the Judgment on EIP is, at base, a challenge to issues of fact and evidence; but (ii), insofar as the applicants cast their proposed grounds of appeal as issues of law they are not arguable.
I. Issue: III: The relevance to fairness of Post Entry Pricing (“PEP”)
The issue
126. Issue III is a variant upon Issues I and II. This time the applicants argue that the level at which post-entry prices settle reflects the result of workable competition. This is likely to be different to the price prevailing where the structural conditions under Issue I existed and/or the EIP under Issue II. On the case advanced by the applicants a PEP is not a single, fixed, price point but could be variable over time. The principle behind a PEP operates upon the premise that once prices have settled they are to be treated as reflecting workable competition and they are, thereafter, free from constraint and might move in any direction, including upwards. On the facts various prices were put forward to the CAT as candidates for PEP ranging up to c.£65. The applicants’ argument upon PEP does not absolve them from a finding of abuse during the Infringement Period for prices above PEP. If their case is made out, the PEP comparator would be exculpatory for prices above Cost Plus up to PEP, and, it could have a major impact upon both the regulatory findings of breach and upon any future civil claim for damages because both Cinven and Hg were owners for the early parts of the Infringement Period before the ASP rose to its ultimate height. The CMA accepted that in principle PEP “might constitute a prima facie valid comparator”. However, it rejected the applicant’s evidence upon the facts upon the basis that PEP: “… continue to be significantly inflated by Advanz’s abusive exercise of market power during the Infringement Period...”: Decision paragraph [5.207(c)(i)]. The CAT agreed: Judgment paragraphs [268]-[281]. The applicants therefore challenge the finding of the CAT that PEP had not yet stabilised.
127. A further issue is whether the relevant benchmark for PEP is (i) the level at which prices settle; or (ii), the level at which prices stabilise in circumstances where they can be said no longer to be contaminated by the prior abuse. The applicants support the former. The CMA argues that it is the latter. The CAT agreed with the CMA.
The position of the CAT on the evidence
128. The CAT did not equate, without more, a stabilised price with a lawful price. It did not say, for example, that after a fixed period of years, say 5 or 7, prices must be deemed in law to be free from contamination. The logic of the CAT Judgment was that it all turned upon the facts and whether or not contamination actually persisted. The CAT however accepted that if there had been evidence that prices had “stabilised” that would have been a “good indication” that prices were no longer contaminated and reflected effective competition: Judgment paragraph [270]. It acknowledged that evidence of shifting market shares and fluctuating prices, such as had occurred between August 2017 and February 2021, could provide “powerful support” for the proposition that by February 2021 prices had reached a workably competitive level: Judgment paragraph [271]. But it found on the evidence that prices had not stabilised and contamination remained. The reasoning of the CAT is set out in Judgment paragraphs [270]-[281]. The main points can be summarised as follows:
(i) The CMA’s evidence of price movements since February 2021 to the date of the appeal showed that prices continued to fall significantly. In the absence of any clear marker to show that the contaminating effect had ceased prevailing prices were arbitrary price points on a downward trend rather than a meaningful benchmark of a competitive price: Judgment paragraph [270].
(ii) The applicants’ experts did not challenge the CMA’s case that the price of Liothyronine Tablets at the time of entry was extraordinarily high. There had been previous price increases of over 6000%. Advanz had intentionally increased prices prior to entry, and the price at entry was exceptionally high compared to any other drug in the sample of 187 in the Oxera study: Judgment paragraph [272].
(iii) The three and a half years which had elapsed after entry was insufficient to ensure a sufficiently competitive price. The Oxera report indicated that, even in an average case of generic price movements after loss of exclusivity, it could take approximately four and a half years for prices to stabilise: Judgment paragraph [273].
(iv) The evidence of Professor Valletti (for the CMA) was that post-entry price movements were unusual and the decline was more gradual than was typical. There was no slashing of prices by Advanz or the new entrants. Advanz tended to price above Teva and Morningside. The gradual rate of price reduction may have been attributable to the fact that, in a market with inelastic demand, a fall in prices would not result in any increase in total volume. The relative “softness” of competition could be attributable to a number of factors, such as the absence of a branded originator product, meaning that there was less of a need for new entrants to price below the originator medicine, or the small size of the market: Judgment paragraph [273].
(v) The 2021 commitments decision of the European Commission in Aspen (Case AT.40394) relied upon by the applicants was instructive but did not assist. It followed an investigation by the Commission into the prices charged by Aspen for certain patent-expired niche cancer medicines. The approach taken by the Commission in relation to PEP was similar to that taken by the CMA. The pre-entry prices were abusively high, and it would take a significant amount of time for post-entry prices to reach competitive levels: Judgment paragraphs [274] - [275].
(vi) The exceptionally high starting-point combined with the unusually slow and continuing decline in prices was an indication that the post entry price in February 2021 was still contaminated and not sufficiently competitive to be a reliable comparator for the purposes of assessing the fairness of any price during the Infringement Period: Judgment paragraph [276].
(vii) Prices in February 2021 were substantially in excess of those for relevant comparables: “…the price of Liothyronine Tablets in February 2021 was a substantial outlier by comparison with the price of other generic drugs”. In particular they were an outlier by reference to:
§ All other Category M drugs in the Oxera Report.
§ Other Category M drugs with a similar market volume.
§ NHS Reimbursement Prices across all Category M drugs.
§ The price of Overseas Liothyronine tablets sold overseas.
§ The pries of Levothyroxine tablets.
The CAT held that the comparisions were “validly made” and “meaningful”. It rejected as inadequate the explanations given by the applicants. It also rejected expert opinion tendered for the applicants that price differentials were explicable by non-price factors. It pointed out that: the tablets were an off-patent unbranded product; patients could switch between manufacturers; there were no research costs or capacity constraints; production could be outsourced; and, the costs of production were not high: See Judgment paragraphs [277]-[278].
(viii) The CAT accepted unchallenged expert evidence tendered by the CMA that Advanz, Teva and Morningside were still earning profits significantly above the profitability to be expected for a generic drug developed in the mid-1950s. Even when prices fell below £70, the EBIT (i.e. earnings before interest and tax) margin remained at 83% or above in each year, and the three suppliers maintained a ROCE of 170%, higher than the 95% of the companies considered in the experts’ sample (Judgment paragraph [279]).
(ix) The CAT did reject one proposition advanced by the CMA expert, namely that the price of Liothyronine would only become workably competitive when it had “…reached an equilibrium close to the direct costs of production”. The direct costs of production are only one component of any calculation of Cost Plus (see paragraphs [34]-[39] above). The CAT (agreeing with the applicants) accepted that this treated prices as workably competitive only once they fell to an equilibrium around direct costs and thereby wrongly effectively equated “workably competitive” prices with “perfectly competitive” prices. The CAT noted that this proposition had been disavowed by the CMA in its closing submission. In paragraph [281] the CAT concluded:
“It is likewise not necessary for us to speculate as to what will happen to the price of Liothyronine Tablets in the future or at what point they will become workably competitive. We have concluded that the February 2021 Post Entry price is not a valid comparator because it is contaminated by the pre-entry abusive pricing, not because prices have yet to converge to an equilibrium around direct costs.”
The CAT did though say, at paragraph [228(3)], that in a competitive market, once fixed costs had been recovered, competition would drive prices “closer” to direct production costs. This is not necessarily inconsistent because there is a subtle, yet important, difference between “close to” and “closer to”.
The applicant’s submissions
129. The applicants argue that the CAT erred in concluding that there was no evidence that prices had yet settled. They say that it was wrong in law to select a test based upon stabilised, uncontaminated, prices, as opposed to observable, settled, prices. The latter amounted to an objective, easily ascertainable, test. On the facts there was no dominance and no collusion and there had elapsed a sufficient period of time during which prices would have settled and be free from contamination. In contrast a test based upon proof of an absence of contamination was a requirement to prove a negative which was inconsistent with the rules on the standard of proof, unworkable in practice and violated the principle of legal certainty.
Analysis
130. The CAT did not err whether in making findings of fact or as to the law. I start with the evidence.
Issues of fact not law
131. First, the CAT did not reject PEP out of hand. It accepted that they could amount to relevant evidence. It did no more than find as a fact that as at the date of the appeal PEP had yet to fall to their competitive floor. It observed that on the evidence prices were trending downwards and it compared the present PEP against a series of relevant benchmarks all of which suggested that there was a considerable way to go before prices hit the competitive resting place. Significantly, the proposed grounds of appeal do not challenge the numerous other reasons relied upon by the CAT, and summarised above (at paragraph [128]), for rejecting PEP including the fact that PEP allowed the applicants to earn very high margins above Cost Plus and remained a distant outlier relevant to an array of valid and meaningful comparators. The gravamen of the complaint boils down to disagreement with the conclusion of the CAT on the single issue that prices were still in a state of downward transition and would fall further. That is a narrow dispute over fact and evidence, and nothing else. There can be no appeal on this basis.
The evidence of continuing contamination
132. Secondly, the submission that PEP reflected workable competition clashes with the facts found by the CMA and endorsed by the CAT. The CMA argued that the market was “soft” and characterised by stickiness of pricing. That described the process whereby, because the market was uncompetitive, the transition from a market characterised by abuse to one characterised by workable competition would be retarded. Advanz, in expectation of new entry, deliberately pushed the price up to an excessive and abusive £247 and the downward progression from that price was “unusually slow”: Judgment paragraph [276]. Various reasons were given for this stickiness. In paragraph [240] one reason was identified as being linked to the Drug Tariff itself:
“240. The Decision found that the stickiness of generic prices was consistent with the fact that when prices are renegotiated, market participants will often take the Drug Tariff as a reference point. The Drug Tariff is itself constructed using a trailing average of market prices so price stickiness is to some extent built into the way prices are renegotiated.”
133. In paragraph [263] the CAT cited the conclusions of Professor Valletti, the CMA expert:
“263. …, the competitive response in a market depends on the behaviour of the market’s players, and they mutually reinforce each other. If an incumbent is not aggressive and does not lower prices significantly, then an entrant has no compelling reason to be particularly aggressive either. If instead an incumbent is aggressive and cuts prices significantly to try to win more sales, then the entrant will also have to respond by reducing prices aggressively, else it would lose sales. Advanz had tended to price above Teva and Morningside. In a market with inelastic demand in particular, the incentives to avoid competition are even stronger, as any fall in prices would not result in any increase in total quantities. In these circumstances, it was not surprising that prices have remained substantially above costs and have declined only slowly. This is an indication of what he termed ‘soft’ competition. This was in contrast to the typical generic path as shown in the Oxera study and the European Commission’s Pharmaceutical Sector Enquiry in which generic entrants significantly undercut the originator from the outset.”
134. Ms Jackson, the expert for Hg, explained, no doubt correctly, that a rational entrant would look to see “… whether competition was too intense” and how tolerant the market was of high prices before entering, which is what happened here. She stated in the Joint Expert report:
“It would not be rational to invest in entry if the expected level of post-entry competition would be too intense to allow a return on entry investment to be made firms may have greater confidence that such prices are achievable post-entry if they have already observed a willingness to pay those prices in the market pre-entry”.
135. The CMA and the CAT set out the facts in terms describing classic oligopoly whereby prices settle at levels above those expected in competitive conditions and which often resemble prices seen in collusive markets, hence the label “tacit collusion”. As set out above at paragraph [65] above the Decision (paragraph [5.257]) recorded that in July 2012, during the acquisition by Cinven of the Advanz business from Hg, a Cinven partner specifically referred to the oligopolistic nature of many generics markets when identifying their attractive features: “… the business’s primary “tail wind” is price increases passed on [to] the payor because of the oligopolistic nature of most segments it operates in, rather than a real growth in volume for each drug…”.
136. Responding to questions from the Court Mr O’Donoghue KC, for Cinven, acknowledged that in the present market it was counterproductive for a new entrant to compete vigorously. That was “… not how competition functions in this market”:
“But because the drug tariff is reported with a degree of latency, there is always a trailing average in the market which itself generates something which could be called stickiness. But again, we would submit that in a workably competitive market, if stickiness means not a death spiral, that is consistent with workable competition. We do not expect firms in workably competitive markets to continuously get out a machine gun to their own foot, that is not how competition functions in this jurisdiction.”
And later:
“… one can see why a supplier doesn't want to get a machine gun under his or her foot and provoke a death spiral in the market.”
137. Mr O'Donoghue KC appears to be correct in saying that self-harming, gun toting, price competition is not how competition functions in this market. But that does not mean that a reluctance to compete vigorously on price is a hallmark of sufficiently (workably) competitive markets, as opposed to oligopolists exploiting ineffectively competitive market conditions. There is no serious challenge to the facts from which the CAT inferred that the PEP relied upon by the applicants did not emerge from a market which, post-entry, was workably competitive. I can set out the relevant facts, as found by the CMA and the CAT, summarily. First, there are facts relating to the market structure itself all of which describe a market which is intrinsically prone to uncompetitive “soft” pricing:
- There were a small number of suppliers.
- Barriers to entry were exceptionally high.
- Prices were transparent due to the Drugs Tariff which meant that suppliers could monitor the price strategies of rivals.
- The product was homogenous and there was no scope for competitors to differentiate their products to win custom. It amounted to old chemistry and did not require any material innovation or improvement.
- There was no commercial incentive to lower prices because demand was fixed and inelastic such that: (i) if prices rose demand would not drop; but also (ii), if prices fell demand would not be increased.
- The purchaser (the NHS) was not the user (the patient) and failed to exercise any countervailing buyer power or impose any effective supervision over prices. It simply paid up regardless.
138. Next, there are facts found relating to the suppliers and their pricing strategies:
- Advanz was a sophisticated entity which deliberately sought out niche generics markets recognising that the inadequacies of (i) their competitive structure and (ii) the regime for regulatory oversight, enabled it to raise prices with impunity.
- Advanz raised prices 63 times over the Infringement Period all with a view to maximising profit.
- Advanz pursued this strategy knowing that it would at some price point likely stimulate entry. When Advanz anticipated actual entry (seemingly in or about 2013) it continued to raise prices sharply (to c.£247 per box in summer/autumn 2017).
- New entrants studied market prices and formed a conclusion as to the profits they could earn upon entry.
- Entry occurred in summer/autumn 2017 when the ASP was at £247, which was c.5000%+ above Cost Plus.
- Post entry there was price leadership (by Advanz) and followership (by Morningside and Teva).
- It would not be normal in such a market for new entrants to come in and price aggressively. There was no incentive to slash prices to the bottom since this would not increase demand and everybody would end up with the same market share but at much lower margins.
- Post entry prices did not fall as quickly as would be expected in a competitive generics market and remained (substantially) above both Cost Plus and relevant comparables.
139. These market features reflect almost ideal conditions in which the effects of a prior abusive price can persist. Since demand is fixed and unresponsive to price, if market participants compete too aggressively on price, they end up sharing the same volume of sales but at lower prices and profits. No one wins. Advanz knew that a strategy of perpetual price increases would trigger new entry yet persisted in pushing the price up as high as possible. Potential entrants watched as prices rose to £247 and chose that price point to enter. As Professor Valletti for the CMA observed the conduct of the incumbent and new entrants is mutually reinforcing. If the incumbent does not compete aggressively in response to new entry those entrants have a good reason to follow the price lead. When the starting price for competition is as high as £247 then, even when prices do fall, there is no incentive for suppliers to compete prices down to competitive levels. Prices can remain contaminated in the long term and even indefinitely.
140. In these circumstances the challenge to the CAT’s conclusion that PEP did not reflect workable competition and were contaminated by the prior abuse, is evidentially unsustainable.
The relevance of contamination as a test in law
141. Thirdly, in relation to the submission that the test is where prices settle and not when they reach a level which is uncontaminated by the prior abuse, the CAT was correct in concluding that the effects of an abuse could linger in a market following loss of dominance. All the experts agreed that following new entry there would be a period of time whilst the market adjusted to competitive conditions and the premise of this consensus was that the effects of an abuse could persist for some considerable period of time after the loss of dominance (see paragraphs [80]-[81] above). No one has argued that there is a principle of law that the effects of abuse cannot outlive dominance. For the reasons the CAT gave the test cannot be at what level prices settle, irrespective of whether the effects of the abuse persist. It is untenable to say that a market must be deemed in law to be workably competitive a fixed number of years after new entry yet accept that the effects of a prior abuse can simultaneously continue to harm the market, however seriously. The two are mutually exclusive.
Contamination and the legal certainty point
142. Finally, the applicants argue that a test based upon stable, uncontaminated, prices violates the principle of legal certainty and as such a test based upon where the price settles is superior. Cinven argued:
“The central legal error made by the Tribunal concerns its misapplication of the concept of “workable competition”. “Workable competition” cannot to be determined by a ‘Goldilocks’ assessment of whether the market has (yet) reached its lowest “stable” price or is free of “contamination” from past high prices. These are inherently vague, subjective, and in material respects unknowable, matters - a point the Judgment ironically makes a virtue of in refusing to “speculate as to what will happen to the price of Liothyronine Tablets in the future or at what point they will become workably competitive”. ([281])
Indeed, it is hard to see how a test based on “stabilised” prices is compatible with legal certainty. What would happen if prices stabilised in future at the levels the Tribunal considered not to be workably competitive? Would the price suddenly become fair? What if prices increased in future? How would the Tribunal ever know whether the terminal price point would ever be reached or by when it had been reached? How would the cause(s) of price rises or decreases be determined? In short, the Tribunal’s assessment is contrary to the principle of legal certainty, which is an essential component of the lawful definition of abusive conduct.”
143. Cinven cited the judgment of the Court of First Instance in Case T-271/03 Deutsche Telekom AG v Commission [2008] ECR II-447 paragraphs [188]-[192] (“DT”) for the proposition that legal certainty was an essential requirement of any test for abuse. This was a “margin squeeze” case where the allegation was that to exclude rivals, Deutsche Telekom (“DT”), the dominant undertaking, maintained a spread between the prices it charged at the wholesale level and at the retail level. Where a dominant undertaking charges an abnormally high wholesale price to rivals (who compete with it at the retail level) but sells its own products at the retail level at a low price rivals may be squeezed out of the market. The Commission found that DT had abused its dominance by such an exclusionary margin squeeze. The Commission conducted an analysis of the costs of DT and held that it was selling at below costs at the retail level. The company argued on appeal that the Commission had failed to carry out a proper investigation because it had not conducted a comparative study of the margins of the main alternative operators on the Spanish market. The Court rejected this argument upon the basis that a dominant undertaking such as DT was aware of its own “situation”, which included its costs, and it could adjust its conduct accordingly upon the basis of that information alone. The Commission did not have to apply a method for determining breach which went beyond this. This approach was consistent with legal certainty because the dominant undertaking did not have to guide its behaviour by reference to the costs of rivals, which it would not generally know:
“188 Next, it must be noted that, although the Community judicature has not yet explicitly ruled on the method to be applied in determining the existence of a margin squeeze, it nevertheless follows clearly from the case‑law that the abusive nature of a dominant undertaking’s pricing practices is determined in principle on the basis of its own situation, and therefore on the basis of its own charges and costs, rather than on the basis of the situation of actual or potential competitors.
…
192 It must be added that any other approach could be contrary to the general principle of legal certainty. If the lawfulness of the pricing practices of a dominant undertaking depended on the particular situation of competing undertakings, particularly their cost structure — information which is generally not known to the dominant undertaking — the latter would not be in a position to assess the lawfulness of its own activities.”
The Court proceeded to find (judgment paragraph [193]) that the Commission was correct to analyse the abusive nature of the applicant’s pricing practises “... solely on the basis of the applicant's particular situation and therefore on the basis of the applicants charges and costs.” This judgment was upheld on appeal (Case-280/08 Deutsche Telekom v Commission ECLI:EU:C:2010:603, 14th October 2010) where (at paragraph [202]) the CJEU affirmed paragraph [192] above. There are other cases to the same effect: see DT paragraphs [188]-[191] and cases therein, and more recently Case C240/22P EC Commission v Intel ECLI:EU:2024:915 at paragraph [312].
144. Arguments about legal certainty are not a sound basis for undermining the conclusion of the CMA and the CAT on Cost Plus. Although the applicants refer to legal certainty to elevate a test of settled prices over that of stable uncontaminated prices, the core argument is that evidence on PEP is superior in quality and probative weight to that of Cost Plus. Even if the applicants were correct that settled prices were superior in terms of evidence to uncontaminated prices this takes them nowhere unless they can also establish that evidence of settled prices prevails over Cost Plus benchmarked against relevant comparables. The principle of legal certainty as described in DT and other case law supports a test based upon evidence of the dominant undertaking's own information and costs, not information about third parties the undertaking would have been unaware of at the time of the alleged abuse, which obviously includes evidence not in existence at the time of the abuse, such as PEP. Insofar as relative evidential weight is concerned the principle indicates that PEP is inferior to Cost Plus, the applicants’ real target.
145. The CAT did not declare PEP to be inadmissible as contrary to legal certainty simply because Advanz was not aware of it at the time of the breach. I agree, as did the CMA and the CAT, that it should not be rendered inadmissible for legal certainty reasons. The judgment in DT is concerned with the types of evidence which can lead to a finding of breach (by a regulator or court) and which might therefore be inculpatory. It does not address other evidence (such as EIP, PEP or MFP) a defendant undertaking might adduce in its defence. The crux of the difficulty confronting the applicants however is that they rely upon evidence that Advanz did not know about, and could never have known about, during the Infringement Period and it is here that the principle of legal certainty is unhelpful to their argument in respect of Cost Plus. As to the argument that a test based upon settled prices is more legally certain that one based upon pricing which is untainted by the prior abuse, there may be little to choose between the two in terms of certainty, both relate to facts the undertaking could never have known about at the time of the infringement. In any event on the applicants’ own evidence PEP is far from being a settled price. The evidence suggests it could be a price in continual flux and the new evidence (see below) shows it presently to be a rapidly rising price.
J. Issue IV - New evidence: The relevance and admissibility of new evidence of PEP sought to be adduced and relied upon by the applicants relating to price movements subsequent to the Judgment of the CAT
The issue
146. Issue IV concerns new evidence sought to be adduced by the applicants. It is relevant to the analysis of PEP under Issue III. At Judgment paragraph [270] the CAT considered that there was an absence of “any clear marker” to show that the “contaminating” effect of the pre-entry 2017 abusive price had ceased by February 2021; a three-and-a-half-year period post entry of competitors was insufficient to assess any contamination of the post-entry generic prices. The CAT also accepted the evidence tendered by the CMA of prices up to July 2022 and data from Cinven concerning pricing up to October 2022 which indicated that prices were still going down: See Judgment paragraphs [257] and [270]. The challenge for the applicants is to show that in 2022 the CAT erred in finding that prices were still going down and had not stabilised upon an uncontaminated basis. Extending the pricing evidence by two years will not necessarily show that the CAT erred in 2022 since it is possible that, even if the new evidence is compelling, prices stabilised after that point in time thereby leaving the CAT conclusion unaffected.
147. This is the context in which the application to adduce new evidence is made. The new data covers the period October 2022-November 2024. The applicants say that there is now over 7 years worth of post-entry pricing and this shows that the £40-£60 post-entry price range was not contaminated by the abusively high price charged in 2017. The applicants point also to the symmetry between the EIP and PEP and argue that the new evidence corroborates the probative value of EIP as an indicator of workable competition. They also contend that the new evidence establishes that Cost-Plus is an inferior evidential benchmark for workable competition.
The application to adduce new evidence
148. The new evidence suggests that in March 2022 the drug tariff reimbursement price was £71.90. This fell to £56.19 in August/September 2022 (when the CAT appeal started) and to £42.48 in August 2023 (when the Judgment was handed down). Between August 2023 and March 2024 it declined to £40.10. However, it then rose sharply so that by November 2024 (just prior to the hearing in this Court) it was at £75.92. Various arguments have been raised by the parties as to the reliability of this data. I propose to take the applicant’s evidence at face value. The disagreements make no difference to the final conclusion. The progression of recent prices over time was summarised in tabular and graphic form as follows:
149. There was, however, no application to adduce this new evidence and the CMA objected. The Court directed the applicants (post-hearing) to prepare and serve a formal application to admit the evidence and gave the CMA an opportunity to respond. The Court indicated that it would hear full argument without prejudice to a ruling that it would make upon formal admissibility in due course. The CMA argues that (i) it is inadmissible since at its highest it concerns an issue of fact over which the Court has no jurisdiction; but in any event (ii), even if admissible it has no or no material probative value which assists the applicants.
150. Advanz Pharma Corp says that the new data is evidence of an absence of contamination:
“Advanz has applied to adduce updated Drug Tariff pricing data for the period October 2022 - November 2024 which provides further evidence that the £40 - £60 post-entry price range was not contaminated by the price charged in 2017. The updated pricing data confirms (a) that there could be no infringement for the period 2009 - 2013 because the prices charged during this time were within the range of workably competitive prices charged since October 2022; (b) the symmetry between the market entry incentivizing prices and the post-entry prices; and (c) that the CMA’s Cost-Plus is a poor benchmark for workable competition.”
151. Cinven argues that the new data is “fundamental” to a series of related issues. It says that: “… the only conclusion is that the CMA’s Cost Plus benchmark is a remarkably poor benchmark for competitive prices” and it “… confirms … that there could be no infringement for the period 2009 - 2013 because the prices charged during this time were within the range of workably competitive prices charged since October 2022”:
“The New Data are fundamental to a series of related issues that underpinned the Tribunal’s conclusions on unfair pricing.
First, they fundamentally cast doubt on the CMA’s Cost Plus benchmark as a valid benchmark for fairness. The CMA’s case was that Liothyronine prices in a competitive market should be at, or proximate to, Cost Plus: see Decision, ¶5.285. The CMA’s Cost Plus figure was £4.94 based on a simple average figure for the period of alleged abuse. The New Data show that DT prices between October 2022 and November 2024 remained 10 to 15 times’ higher than the CMA’s Cost Plus figure. Moreover, the trend in the DT price since March 2024 is distinctly upwards, having risen from £40.10 in March 2024 to £75.92 as of November 2024. In these circumstances, the only conclusion is that the CMA’s Cost Plus benchmark is a remarkably poor benchmark for competitive prices, even allowing for the fact that, as noted, ASPs for generic drugs are typically 10-20% below the DT price. Since the CMA has no other specific benchmark to show unfairness, it follows that it has not discharged its burden of proof.
Second, the New Data cast serious doubt on the basis for the Tribunal’s rejection of Liothyronine post-entry prices (“PEPs”) as a valid benchmark. As the Court is aware, the alleged infringement ended on 31 July 2017. Actual and potential competition from firms other than Advanz then immediately occurred, leading to material price falls in PEPs, substantial market share shifts, and the generation of substantial customer benefits in the form of new types of Liothyronine products (e.g., different doses, presentations, allergic versions).”
The law on admissibility
152. This Court has to determine, first, whether the new evidence meets the test for admissibility, and secondly, if it does, whether it is sufficient to cast into doubt the findings of the CAT and, if that is the conclusion of the Court, what the practical consequences are. As set out below the question of admissibility includes an evaluation of the quality of the evidence so that there is an overlap between admissibility and the assessment of the evidence if admissible.
153. The previous Rules of the Supreme Court distinguished between new evidence in existence during the hearing below but not adduced, and evidence which came into existence after the date of the hearing. R.S.C. Ord. 59 r.10(2) provided:
“The Court of Appeal shall have power to receive further evidence on questions of fact … but, in the case of an appeal from a judgment after trial or hearing of any cause or matter on the merits, no such further evidence (other than evidence as to matters which have occurred after the date of the trial or hearing) shall be admitted except on special grounds.”
154. The “special grounds” test was encapsulated in the famous Ladd v Marshall test. Evidence was admissible if (in summary): (i) it could not have been obtained with reasonable diligence; (ii) it would probably have an important bearing on the result of the case; and (iii), it was credible. Guidance has been given on the probably important limb of the test in Ladd v Marshall in the judgment in Re T (Fresh evidence on appeal) [2024] EWCA Civ 1384 at paragraph [33] where “probably” was held not to refer to a balance of probabilities but whether there was a “real possibility” that if the evidence was admitted it would have an important influence upon the outcome of the proceedings.
155. Case law established that fresh evidence, not in existence at the time of the hearing, was admissible if it “falsified” the basis of the first instance judgment. Such evidence did not imply fraud or culpability but went to a fact that was important to the ratio of the judgment which, in the event, turned out to be incorrect. Such evidence was admitted as a matter of discretion with the Court balancing finality of litigation with the overall fairness of the result. The intrinsic quality of the new evidence was important. Uncontentious and objectively verifiable evidence was more likely to be admitted: See e.g. Murphy v Stone-Wallwork (Charlton) Ltd [1969] 1 WLR 1023; and Mulholland v Mitchell [1971] AC 666.
156. RSC Ord 59 r10(2) was replaced by CPR 52.21(2) which makes no formal distinction between new evidence existing at the time of the hearing below and evidence of changed circumstances post-trial. It provides for a discretion on the part of the appeal court to receive new evidence. It states: “Unless it orders otherwise, the appeal court will not receive ... (b) evidence which was not before the lower court”.
157. This Court continues therefore to have the right to order the admission of new evidence whether or not it was in existence at the timing of the hearing below. The CPR cannot, however, override statute and since the jurisdiction of this Court in a competition case is limited on substantive issues to a point of law the new evidence must bear upon such a point. A power to admit new evidence cannot be used to turn an appeal on a point of law into an appeal on issues of fact: See e.g. E and R v. Secretary of State for the Home Dept. [2004] EWCA Civ 49 (“E and R”) at paragraph [23(i)].
158. In E and R the Court of Appeal summarised the test for admissibility at paragraph [23(ii)], melding the CPR with Ladd v Marshal:
“… first, that the fresh evidence could not have been obtained with reasonable diligence for use at the trial; secondly, that if given, it probably would have had an important influence on the result; and, thirdly, that it is apparently credible although not necessarily incontrovertible.”
159. In written submissions the CMA accepted that a mistake of fact going to fairness could amount to an error of law based upon the notion that in some cases the parties shared an “interest” in cooperation to achieve the correct result. It cited E and R paragraph [66]:
“In our view, the time has now come to accept that a mistake of fact giving rise to unfairness is a separate head of challenge in an appeal on a point of law, at least in those statutory contexts where the parties share an interest in co-operating to achieve the correct result. Asylum law is undoubtedly such an area.”
In the light of this the CMA made the following important statement:
“31. The CMA accepts that competition law is such an area: it is an area where the CMA has a shared interest in ensuring that decisions are taken on the best information and on the correct factual basis: see further R (Iran and others) v Secretary of State for the Home Department [2005] EWCA Civ 982 (“Iran”), para 30…. In the circumstances, if a mistake of fact giving rise to unfairness is identified, it is a ground of appeal which may be advanced under section 49(1) of the Competition Act 1998.”
160. The CMA caveated this by arguing that the scope for such appeals was exceptional and limited to cases of unfairness citing E and R paragraph [66]:
“Without seeking to lay down a precise code, the ordinary requirements for a finding of unfairness are…. First, there must have been a mistake as to an existing fact, including a mistake as to the availability of evidence on a particular matter. Secondly, the fact or evidence must have been “established”, in the sense that it was uncontentious and objectively verifiable. Thirdly, the Appellant (or his advisers) must not been have been responsible for the mistake. Fourthly, the mistake must have played a material (not necessarily decisive) part in the Tribunal's reasoning”.
161. The applicants cite R (Iran) v Secretary of State for the Home Department [2005] EWCA Civ 982 (“R (Iran)”) for the proposition that the Court recognises that certain challenges based upon facts can amount to errors of law. There the Court (paragraph [9]) summarised the factually based arguments which gave rise to points of law most frequently encountered in practice and it is clear that points of law were not confined to cases of unfairness:
“i) Making perverse or irrational findings on a matter or matters that were material to the outcome (“material matters”);
ii) Failing to give reasons or any adequate reasons for findings on material matters;
iii) Failing to take account and/or resolve conflicts of fact or opinion on material matters;
iv) Giving weight to immaterial matters;
v) Making a material misdirection of law on any material matter;
vi) Committing or permitting a procedural or other irregularity capable of making a material difference to the outcome or the fairness of the proceedings;
vii) Making a mistake as to a material fact which could be established by objective and uncontentious evidence, where the Appellant and/or his advisors were not responsible for the mistake, and where unfairness resulted from the fact that a mistake was made.”
162. The applicants submit that the new evidence is relevant to an error of law, admissible and probative:
(i) The CAT relied upon a short time sequence of price data. It concluded that the period was too short to form a conclusion that the prices had stabilised. There was now 7 years worth of evidence upon which to form a conclusion and this established that prices had settled and could be seen to be unaffected by any lingering contamination. That went to a material fact and amounted to an error of law within paragraph (vii) of the categories of error of law in R(Iran). There was a mistake as to a material fact which could now be established by objective and uncontentious evidence where the applicants were not responsible for the mistake and (insofar as it was necessary to establish) unfairness resulted from the fact of the mistake.
(ii) The Court should exercise its discretion to admit the evidence. Infringements of competition rules are quasi-criminal and binding as to liability in subsequent follow on claims for damages and in director disqualification proceedings. This heightened the importance of the Court admitting the evidence. This was, in any event, an area of law of public importance, as the CMA acknowledged.
(iii) It was also pointed out that the CAT had noted (Judgment paragraph [281]) that it was “not necessary for us to speculate as to what will happen to the price of Liothyronine Tablets in the future or at what price they will become workably competitive.” Lord Wilberforce in Mulholland however commented that the interests of justice may dictate that “the court should not speculate where it knows” (page [679F]).
Analysis
163. As a general proposition I agree with the applicants and the CMA that there is, in competition cases, a strong public interest in arriving at the correct decision. Regulatory interventions can shape the very future of markets, to the detriment of all if they are ill-founded. There is a real premium upon getting it right. Accordingly, if evidence exists indicating that a decision is incorrect that is a matter which will weigh in the discretionary scales tilting in favour of admissibility. But that is not the end of the story. Many, if not most, applications to adduce new evidence arise before the substantive appeal hearing. Here the application arose at the conclusion of the hearing and the Court heard full argument on all matters arising including as to whether the proposed grounds of challenge to the Judgment on PEP amounted to issues of fact or law.
164. There are a series of reasons why I conclude that the new evidence does not assist the applicants.
165. First, the objection is as to evidence, not law: See paragraph [131] above. The central issue of law which arose in relation to PEP concerned whether the test was settled prices or, alternatively, stabilised prices at a level where there was no evidence of contamination. The new argument however takes as its starting point that the evidence must address the issue of contamination, so the new evidence is not relevant to the dichotomy between settled and stabilised, uncontaminated, prices. As to the facts the CAT found that prices remained contaminated, and it took account of evidence up to October 2022 which coincided with the hearing of the appeal before the CAT: See Judgment paragraphs [257] and [270]. The new evidence is of the same type as was adduced before the CAT and simply prolongs a sequence of temporal markers. It goes to the conclusion of the CAT that as of 2022 prices had not yet fallen to stabilised, uncontaminated, levels. In the context of the case as a whole that was a finding of fact, and the new evidence seeks only to challenge that fact finding. It does not go to a point of law.
166. Secondly, even standing alone the evidence is not capable of having an important (potentially exculpatory) influence upon the result, applying the test set out in paragraph [154] above. The most cogent inference to draw is that the new evidence is consistent with the findings of the CMA and CAT that observable prices reflected “soft”, ineffective, market conditions where the effects of the abuse persist. Even c.£40, the lowest point to which drug tariff reimbursement prices fell as recorded in the new evidence, is substantially out of kilter with Cost Plus and all the comparables relied upon by the CAT. Further, as is recorded in the table above, between March and April 2024 the price rose, suddenly, by over 50% (from £40.10-£62.32) and then between September and October 2024 the price rose again this time by about 25% (from £60.43-£75.92). In the course of about 8 months (March-November 2024) the price came close to being doubled. The thrust of the applicant’s argument is that the longer period of time it can now refer to demonstrates that prices have stabilised and are not on a continual downward trajectory. It is said that this undermines the CAT conclusion that prices had not yet stabilised in 2022. But the new evidence is startling and shows not that there is flattening or stabilising after a period of decline (for instance at c.£40) but that prices are now on a steep ascent. The fact that prices are going up dramatically seems to me a clear indicator that something in the market remains wrong. The pattern of price movements is consistent with oligopoly pricing whereby ineffective market structure enables competing suppliers to set prices at uncompetitive levels which perpetuate the effects of prior abuse. I make no formal findings to this effect since the evidence has not been properly tested. What I do reject is the contention that the only, or even a, sensible explanation of the new evidence is that it undermines the CAT’s finding of fact that there was still contamination.
167. Thirdly, even though the applicants argue that this evidence points unequivocally in their favour, it has not been tested in either regulatory proceedings or in the crucible of a trial on the merits before the CAT. There is no accompanying disclosure explaining why prices have gone up so dramatically. There is no evidence (including through cross examination) as to the present pricing strategy of Advanz and whether its aim is to push prices up even higher in the expectation that others will follow. There is no explanation of how the present market has changed. The CMA points out that the categorisation of Liothyronine Tablets under the Drug Tariff has changed (from Category M to Category A) with the consequence that the method for setting prices for the drug has also changed and that this coincided with the significant but unexplained price increase from £40.10 to £62.32 in March/April 2024. Importantly, the new evidence has not been considered by the experts. An important feature of the merits appeal was that pricing data was subjected to detailed, iterative, analysis whereby the experts were required to meet, discuss and seek common ground on relevant issues. This was a process of value to the CAT in shaping its analysis. The applicants do not suggest that this Court should conduct any sort of a “trial” of this new evidence. The applicants mooted the possibility (to overcome these problems) that the issue be remitted to the CAT. This would have arisen for consideration had I concluded that the evidence (i) was admissible and went to a point of law; and (ii), was of a potentially exculpatory quality such that it would be unfair not to remit the matter for proper consideration. Those conditions are not met.
168. Fourthly, but importantly, the new evidence goes to but one slice of but one reason out of the many given by the CAT for rejecting PEP: See the summary at paragraph [128(i)-(ix)] above. Even were this new evidence to be material to that one point it does not alter the analysis of the CAT in relation to the other reasons given for rejecting the submissions of the applicants, for example in respect of comparables.
169. Finally, I should mention one point that arose upon which it is not sensible to express a view. One possibility in relation to new evidence which an undertaking considers materially affects the outcome of a prior decision, is to submit that information to the CMA and invite the CMA to review the evidence and then withdraw the decision. The CMA would then have to decide how to deal with it. During the oral hearing the CMA accepted that this was a possibility but, subsequently, in written submissions it cast doubt upon the possibility. Whatever might be the position it does not arise in the present case, and even if it had existed as a possibility, it does not affect the jurisdiction of this Court to evaluate the evidence and rule upon it in an appropriate case.
170. In conclusion, I dismiss the application to adduce new evidence as not raising an issue of law, but had I admitted the evidence I would not have found that it supported the applicant’s case but, to the contrary, prima facie, it was consistent with the conclusion of the CAT in relation to PEP that prices across the entire market remained contaminated, causally, by the prior abuse.
K. Issue V - MFP: The relevance to fairness of the prices that would have been charged in a competitive market with multiple suppliers needing to recover their fixed costs over lower volumes (“multi-firm pricing” or “MFP”).
The issue
171. Under Issue V the applicants argue that since in a workably competitive market there will be multiple suppliers the unit costs per supplier would be higher because firms would need to recover costs over lower market shares. Initially, before the CAT the applicants argued that this was relevant to Cost Plus. However, as the CAT recorded in Judgment (paragraph [139]), by the end of the hearing the applicants had accepted that Cost Plus was appropriate for determining whether price were excessive but argued that MFP was still necessary to an evaluation of fairness. Reliance was placed upon the opinion of Advocate General Wahl in Case C-177/16 Autoriesbu un Komunicesanas Konsultaciju Agentura / Latvijas Autoru Apvieniba v Konkurences Padome (6th April 2017) EU:C:2017:689 ("Latvian Copyright") for the proposition that a test of fairness always entailed the use of hypothetical modelling, of which multi-firm analysis was an example.
172. The CMA decided that a “multi-firm” adjustment was “neither necessary nor appropriate”. There was no need or requirement to use a hypothetical benchmark or any other artificial construct. A Cost Plus assessment based upon the costs actually incurred by Advanz as the monopoly supplier during the Infringement Period was possible and preferable to the use of notional costs in a hypothetical multi-player market which did not exist during the Infringement Period: Decision paragraphs [5.201] and [5.355]-[5.359].
The CAT Judgment
173. The CAT agreed with the CMA. Throughout the Infringement Period, Advanz was in a monopoly position. A multi-firm scenario was hypothetical and divorced from reality and the CAT had not been referred to any case in which a multi-firm adjustment was made to a Cost Plus calculation or had been treated as relevant to fairness:
“222. A multi-firm adjustment can make a significant difference to the end result. This is illustrated by the following table in Dr Bennett’s report (which uses the CMA’s cost assumptions):
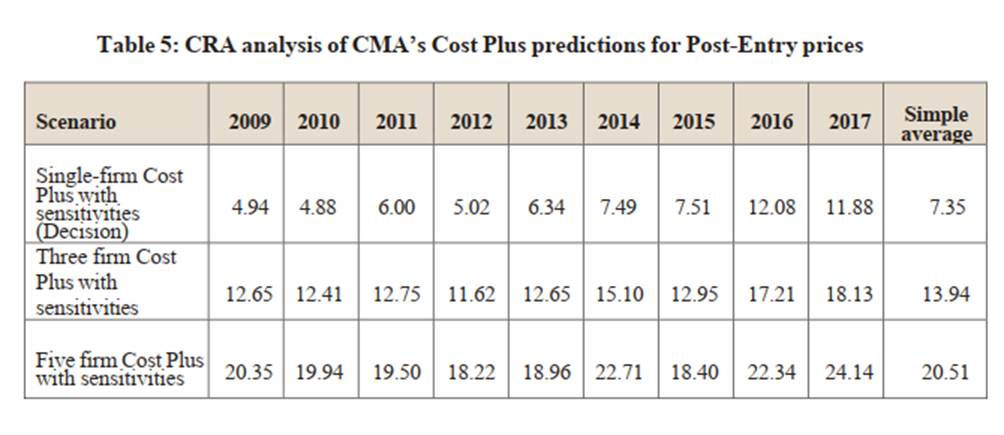
223. Extending these examples further, with ten firms the Cost Plus would be £37.50 and with 70 firms £247.88
224. By the end of the hearing, the Appellants accepted that a single-firm Cost Plus calculation was appropriate for the purposes of the Excessive Limb of the United Brands test but maintained that a multi-firm adjustment was necessary for the purposes of the Unfairness Limb.
225. In the Decision, the CMA contended that a multi-firm adjustment was inappropriate both as a matter of economic logic and from the perspective of effective competition policy enforcement, for the following reasons.
(1) The multi-firm adjustment is flawed because it is premised on the incorrect assumption that the CMA’s intervention threshold must leave room for entry by other competitors. In a market which is characterised by high entry costs relative to market size, as is the case for Liothyronine Tablets, applying a multi-firm adjustment would defeat the purpose of the law, which is to require companies with significant market power to exercise restraint.
(2) Permitting an incumbent to charge a multi-firm price in such a scenario would be perverse in that it would enable an incumbent to recoup as pure economic profit the modelled costs of operating in a hypothetical multi-player market. This would result in significant harm to consumer welfare. The adjustment is also divorced from economic reality since the significantly higher prices produced by the adjustment bear no relationship to the incumbents’ costs or the products’ economic value.”
174. The CAT then proceeded to endorse the reasoning of the CMA (Judgment paragraphs [227]–[229]):
(i) The use of the multi-firm Cost Plus was not an appropriate tool for assessing the fairness of a dominant undertaking’s prices since it would enable an incumbent to retain as pure profit the costs of operating in a hypothetical multi-player market even though the higher prices produced by the adjustment were unrelated to the reality of the market in which the incumbent was a monopolist.
(ii) Where there were high entry barriers (as in the present case) the incumbent could profit from those barriers and charge prices unrelated to its own costs or to the product’s economic value.
(iii) It was common ground between the experts that the multi-firm Cost Plus model was not informative of the price that would be obtained under conditions of workable competition. It did not model competitive prices or predict the price that would be obtained under competitive conditions. The calculation depended upon assumptions as to the number of competing firms. The competitive price could be higher or lower than the multi-firm Cost Plus. Furthermore, the costs taken into account in the model included the fixed costs of entry but those costs had no long-term effect upon a firm’s pricing. Once firms had recovered those costs, competition would drive prices “closer to” the direct costs of production.
(iv) Case law (United Brands/Phenytoin) did not compel the use of any particular benchmark and there was in law no requirement that fairness had to be determined by reference to prices in a multi-firm scenario.
(v) Even were a multi-firm adjustment to be made, Advanz’s actual prices materially exceeded it. If three hypothetical firms had charged Advanz’s actual prices (£20.80 on average in 2009 rising to £247.77 on average in 2017) they would each have made economic profits of £8.16 per pack in 2009, rising to £229.88 per pack in 2017. These equated to a differential above Cost Plus adjusted for multi-firm of around 65% in 2009, rising to around 1,285% in 2017. This adjustment would significantly understate the actual differential which Advanz earned above its costs (900% in 2009, rising to 2,434% in 2017) because it did not incur the modelled costs of operating in a hypothetical multi-player market.
Analysis
175. Before this Court the applicants repeated the submissions made below: MFP reflects pricing in workable competitive markets and the use of hypothetical modelling is mandatory. I do not accept that the CAT erred. I endorse the reasoning of the CMA and the CAT and would add very little. There is no requirement in law to use hypothetical modelling. The Court in Phenytoin addressed the opinion of the Advocate General and the judgment of the CJEU in Latvian Copyright at length (see paragraphs [78]-[86] and [118]-[125]) and made clear that there had to be “a” benchmark but that there was no fixed rule that hypothetical models or benchmarks were mandatory. It rejected the argument that the Opinion of the Advocate General in Latvian Copyright required the use of any such hypothetical benchmark price: see paragraph [124]. Further, there is no authority indicating that a MFP model should be used or that if a regulator having considered the evidence rejects it as a matter of discretion as being of less probative value than some other methodology (such as Cost Plus) or category of evidence, that this amounts to an error of law on the part of the regulator as opposed to the legitimate exercise of evaluative judgment over the evidence. Finally, relative to Cost Plus and actual, real life, comparables pertaining during the Infringement Period, MFP is far less legally certain as a test: See paragraph [202] below.
L. Issue VI - Portfolio Pricing: The relevance to fairness of pricing designed to generate high profits in relation to one product which can then be used to subsidise other products.
The issue
176. Issue VI concerns “portfolio” pricing. The applicants did not develop this argument during the hearing, but it was dealt with in written submissions. This describes the situation where an undertaking charges high prices on one product (x) in order to use the surplus to subsidise some other commercial activity such as low prices on a different product (y), including outside the product market for x. It is argued that the fairness of an undertaking’s prices must be measured by reference to pricing for the entire portfolio of products in question i.e. x and y.
177. An expert witness for Advanz opined that restricting the returns on individual products removed the ability of pharmaceutical companies to cross subsidise other products and would lead to price increases on previously subsidised medicines. An analysis of Advanz’s assessed profit levels on the supply of the entirety of its NHS medicines, using the normal NHS/PPRS profit assessment methodology, was undertaken. This indicated that the profits of Advanz overall were below the maximum allowed profitability under the PPRS. A witness of fact for Advanz, Mr Beighton, claimed that portfolio pricing was the norm in the pharmaceutical industry and was recognised by the PPRS and by the NHS in its approach to establishing a fair price for generic drugs. He referred to a slide prepared by Advanz’s lawyers for the CMA in 2018 which did not relate to the Infringement Period and which spoke in the abstract about the utility of portfolio pricing.
178. It is argued that the CAT erred when its agreed with the CMA that: (i) portfolio pricing was not an appropriate measure or benchmark against which to measure the fairness of the prices actually charged; and (ii), there was no evidence showing that Advanz ever adopted a portfolio pricing approach.
179. As to the law the CMA contended that in the judgment in Napp (ibid) the CAT ruled out, definitively and as a matter of principle, the relevance of portfolio pricing. Undertakings have a special responsibility for each product in respect of which they hold a dominant position, and it is no defence to otherwise unfair pricing in market x that the excess is used to subsidise prices in the different market y. In Napp the CAT held that:
“Napp's whole argument based on “portfolio pricing”, impermissibly directs attention away from the specific product market which we are required to consider when deciding whether there is an abuse of a dominant position under section 18 of the Act. In our view, it is not appropriate, when deciding whether an undertaking has abused a dominant position by charging excessive prices in a particular market, to take into account the reasonableness or otherwise of its profits in other, unspecified, markets comprised in some wider but undefined “portfolio” unrelated to the market in which dominance exists.”
The applicants say that all the CAT did in Napp (judgment paragraph [219]) was to reject the submission on the evidence, not in principle.
Analysis
180. There are three reasons why I reject the proposed ground of appeal.
181. First, the evidence, a representative portion of which has been summarised at paragraphs [59]-[70] above, reflects a laser sharp focus on the part of Advanz upon generating profits, to put it bluntly, for its own sake and not for some other acceptable purpose. Its commercial objective was not to generate funds to invest, for instance, in groundbreaking medical research or even to subsidise other low priced products. The CAT was correct to reject the evidence, such as it was, suggesting that Advanz ever followed a strategy of portfolio pricing. The short answer to the proposed ground of appeal is that it amounts to a challenge to a finding of fact by the CAT on the evidence tendered during the appeal. In the present case the CAT made clear that its decision was made on the facts. It said only this of the judgment in Napp:
“It is not necessary for the Tribunal to determine whether the judgment in Napp is to be read as establishing a general principle that portfolio pricing can never be relevant to the question of whether a price of a particular product is abusive. We agree with the CMA that the portfolio pricing issue is a red herring in this case given the absence of evidence that Advanz was actually setting the price of Liothyronine on a portfolio basis, rather than increasing the price as a means of profit maximisation without reference to other products.”
182. Secondly, even assuming that portfolio pricing is capable of amounting to a proper justification for an otherwise unfair price, there would have to be some evidenced correlation between the excess said to amount to unfairness and the subsidised price and some explanation as to how and why the subsidised price was beneficial for consumers and not artificially harmful to competition. If the excess is 100 units but only 5 units are devoted to a valid portfolio purpose that could not exonerate the residual 95. In this case no attempt at any form of correlation or justification has occurred. As was explained in Phenytoin, there is an evidential burden upon the undertaking being investigated. The evidence of a causal link between a surplus and cross-subsidisation of some other product will be in the possession of the undertaking concerned. It is not unreasonable to expect it to adduce that evidence if it wishes to make a case based upon portfolio pricing: see paragraph [118] above and the citation of paragraphs [165] and [166] of the judgment of the CJEU in Generics (UK), which would apply here.
183. Thirdly, as to the law, were a price that was excessive to become justifiable (and “fair”) simply because, in the abstract, the excess might (theoretically) have been used for some other usage in the public interest, a veritable cart and horses would be driven through the prohibition on unfair pricing. As was observed in Napp, portfolio pricing involves the proposition that conduct which is otherwise abusive in market x becomes non-abusive because it is used to lower prices in market y. The premise behind that judgment was that this was objectionable. In many cases that might be correct. There must at least be a serious doubt about the legality of a dominant undertaking using otherwise ill-gotten gains derived from market x to subsidise prices in market y, where it is not dominant, and where those subsidised prices might enable it to compete in an artificial manner with competitors there.
184. On the other hand, I would not go so far as to say that portfolio pricing is inevitably irrelevant. The CAT in this case left this as an open question. In Phenytoin the Court of Appeal was at pains to avoid a situation whereby any particular justification could, in advance of an actual case where the facts would be examined, be ruled either definitively in or out. Take for example the paradigm illustration sometimes provided of a pharmaceutical company engaging in expensive and risky research across a range of products only some of which (impossible to predict in advance) might turn out to be commercially viable. There may well be a cogent public interest in permitting that entity to earn a higher margin on the rare successful (usually patent protected) product in order to enable it to continue to invest in research in other potential products. In such a case a regulator might accept that, if there is dominance, a “fair” price on the successful product might be materially above Cost Plus. Considerations of this type might need to be reflected in a fairness analysis, whether as part of the “plus” calculation, or in relation to “value”, or in some other way: See generally Phenytoin paragraphs [153]-[173] on patient benefit and economic “value”. The justification for portfolio pricing outside of the research based pharmaceutical sector might however be weaker .
185. In this case the CAT rejected the case of Advanz on the evidence. It was justified in doing so. No appeal can lie against this finding of fact.
M. Issue VII - Acquiescence: The relevance to fairness of the fact that the NHS did not object to pricing subsequently found by the CMA to be abusive.
The issue
186. Under Issue VII it is argued that:
(i) The acquiescence of the NHS means that the pricing conduct of Advanz was not unilateral as is required as a condition for a finding in law of dominance and abuse.
(ii) There is a general principle of acquiescence laid down by the CAT in Genzyme Limited v Office of Fair Trading [2004] CAT 4 (“Genzyme”) which applies to the NHS when purchasing medicines. There are three conditions to be satisfied for the principle to apply: (a) that the NHS was aware of the price increases during the Infringement Period; (b) that the scale of prices charged to the NHS reflected policy matters which the NHS might properly address; and (c), that the NHS made no complaint or criticism of those prices. Advanz argued that these conditions were met on the evidence and, applying the Genzyme test, there was acquiescence.
(iii) The principle of acquiescence is is akin to the tortious defences of contributory negligence/failure to mitigate which are “potentially open” to defendants in competition law civil claims: see e.g. Secretary of State for Health v Servier Laboratories [2020] UKSC 44 at paragraph [7] (“Servier”).
The Decision
187. The Decision (paragraph [5.245]) summarises the argument put forward to the CMA by Advanz about acquiescence and the CMA response which was to reject the argument on the facts. The Decision explained that Advanz had argued that where there was acquiescence (as defined) then the conduct of the dominant undertaking could not be said to be “unilateral” and hence could not be an abuse:
“Advanz also argues that there can have been no abuse of a dominant position in this case as it did not act unilaterally, but rather the prices of Liothyronine Tablets were the outcome of agreement between Advanz and the DHSC/NHS.”
188. Annex 6.2 to the Decision sets out the evidence refuting the submission. The CMA added (Decision paragraph [5.245]) that the argument on acquiescence provided no insight into the economic value the NHS attached to Liothyronine Tablets or its willingness to pay Advanz’s prices:
“… it is not the case that the DHSC/NHS considered Advanz’s price increases to reflect any enhanced value in the product, that it ‘approved’ Advanz’s prices as reflective of the economic value of Liothyronine Tablets, or that it made an informed decision not to intervene in those prices for that (or any other) reason. The CMA therefore rejects Advanz’s argument that ‘The DH’s/NHS’s willingness to pay and the informed decision it took not to intervene reflects the economic value that the DH/NHS ascribes to LIO.”
The findings of the CAT
189. In relation to Genzyme, the CAT rejected the argument that the judgment established a principle of acquiescence. It pointed out that the conduct in that case said to have been acquiesced to had never been found by the CMA to be an abuse in the first place: see Judgment paragraphs [401] and [408]-[409]. So, even if, evidentially, there had been acquiescence by the NHS it was not to conduct that was legally abusive and hence there was nothing which the acquiescence could neutralise as an abuse.
190. The CAT also held that in any event there was no acquiescence on the facts: Judgment paragraphs [413]-[418]. Such material as Advanz put to the NHS was “perfunctory”. It did not enable the NHS to make an “informed assessment”. Price increases were never evaluated by the NHS for “substantive justification”. No information was ever provided to the NHS about the reasons for price increases. There was never any agreement or understanding between Advanz and the NHS within or outside of a price notification process that increases were approved upon the basis that they were necessary to improve security of supply or other compliance issues. There was no acknowledgement that price increases were necessary under any so-called portfolio approach to pricing. In paragraph [418] the CAT concluded:
“In summary, the Tribunal concludes that Advanz did not intend to, and did not, provide the DHSC/NHS with sufficient information to make an informed assessment of the price increases and Advanz could not reasonably have inferred that the DHSC/NHS approved of the price increases.”
Analysis
191. The applicants did not address this issue in oral argument, but they did in writing. They repeat the submissions made to the CMA and the CAT. I do not accept the arguments.
192. First, on the evidence there was no acquiescence for all the reasons the CAT found. The avowed strategy of Advanz was to go off radar and avoid the gaze of the regulators. The internal documents demonstrate that this was integral to the price optimisation strategy. This is inconsistent with any notion of there ever having been acquiescence. The core challenge on this ground is to findings of fact. Even if the applicants were right on the law, they would still have to overturn the CAT’s conclusion on the evidence. I would add a modest caveat. If there had in fact been express acquiescence following full and frank disclosure of all facts, that might have some role to play, as part of the overall evidential matrix, in the evaluation of fairness. Had the NHS, fully appraised, indicated that for a rational policy reasons high prices were justifiable this might, at least arguably, have been a consideration the CMA could have taken into account in its analysis (portfolio pricing by research based companies might be an example). It might, for instance, have been reflected in “value” or in fairness. That is as far as it goes. The NHS has not, in this case, ever advanced any such reason, so the issue does not in the event arise for consideration.
193. Secondly, the argument that acquiescence of the NHS rendered unilateral conduct multilateral is unsustainable. The concept of unilateralism flows from the legal definition of dominance as the ability to act independently on the market where independence means free from constraint, including from pressures imposed by customers or rivals. Where an undertaking is so constrained, for example by its customers, it lacks the ability to act unilaterally or independently, and it cannot be dominant in law. In the present case the CAT rejected a submission of Advanz that the countervailing buyer power of the NHS was such as to negate dominance and this rejection vindicates the finding by the CMA and the CAT that Advanz had the power to act independently ie unilaterally: Judgment paragraphs [351]-[391]. There is no application to appeal this finding. Insofar as the argument extends to saying that because the prices were contractual, and hence consensual, they cannot be unilateral, Section 18 CA 1998 categorises conduct as an unfair abuse even though, in almost every case, the unfair term or price has been agreed to by a contractual counterparty and is in that sense not unilateral. But this is beside the point. The abuse arises from an imbalance of power between the parties which gives the dominant party the power to impose unfair terms and it is this which is accounted for as “unilateral”, a characterisation not negated merely because economic power is exercised through medium of contract.
194. Thirdly, for the reasons given by the CAT (see paragraphs [189] above) the judgment in Genzyme does not create any overarching test of acquiescence. Insofar as it is contended that general acquiescence or passivity by NHS statutory bodies (falling short of countervailing buyer power) can expunge the abusive nature of otherwise unlawful conduct, I do not understand the argument. At the level of first principle the acquiescence of a body not tasked by Parliament with the enforcement of competition law cannot oust the power and duty of an agency specifically tasked by Parliament with the job of enforcing that law. In any battle of competence, the agency allocated responsibility for enforcing the law by Parliament necessarily and inevitably wins out. If it were otherwise, then regulatory failure on the part of the NHS would leave consumers and markets unprotected and the competent regulator impotent to act.
195. Fourthly, I do not accept that tortious principles of contributory negligence and/or mitigation are relevant as an analogy or otherwise. In Servier the Supreme Court did no more than note, in passing, that Servier, who was defendant in a claim by the Secretary of State for damages, had been allowed by the High Court to amend its pleadings to allege that the Secretary of State had been contributorily negligent in agreeing to pay high prices and by not promoting cheaper generic alternatives: see Servier paragraphs [6] and [7]. There was no suggestion from the Court that any such defence would succeed. The defence seems improbable. In breach of its special responsibility to act fairly Advanz designed a long term strategy to exploit, with single minded determination, what it perceived to be a systemic regulatory weakness or lacuna in oversight. The special responsibility imposed by the law on dominant undertakings entails a duty not to exploit market power to the fullest degree, but to moderate behaviour so that it apes or reflects that which is open to an undertaking in a competitive market. To say that a victim failed to prevent itself from being deliberately exploited in this manner, and that this amounts to a defence, risks turning the pivotal doctrine of special responsibility upon its head. I can identify no principle of competition law, or policy, and no authority, which treats as exculpatory or ameliorative of liability the failure of the victim to protect itself from the abuse .
N. Issue VIII: The burden and standard of proof: Did the CAT disregard the burden and standard of proof in relation to pricing issues?
The issue
196. Issue VIII concerns the quality of the different strands of evidence. The applicants point out that it is trite that the CMA bears the burden of proof which is high given the quasi-criminal nature of the penalties imposed (Phenytoin (ibid) paragraphs [136]-[140] and case law cited thereat). When market prices reflect workably effective competition this amounts to evidence of the highest quality and it is then wrong in principle for the CAT to endorse a test reliant upon Cost Plus, which rests upon inferior evidence unconnected to workable competition. The CAT also erred in treating the CMA’s Cost Plus “… as the exclusive basis for upholding the Decision’s finding of unfair prices instead of applying a multi-benchmark approach and applying different weights to those benchmarks in accordance with…” (emphasis added). Cinven points to the enormous “… gulf between the CMA’s Cost Plus benchmark and the PEPs, EIPs, and other benchmarks relied upon” by the applicants. It says that its evidence of post-entry pricing demonstrate convergence which heightens its evidential value and undermines the use of Cost Plus which also lacks legal certainty because it fails to indicate how prices might react in a workably competitive market.
197. The particular points raised about evidence are that the CAT erred in law in that:
- It relied upon Cost Plus which is based upon evidence generated in a monopoly whereas the applicant’s evidence is superior being based upon workable competition.
- The analysis of Cost Plus was unsupported by corroborating evidence.
- The CAT ignored the fact that the applicant’s evidence was convergent which entitled it to greater probative weight.
- Cost Plus lacks legal certainty.
I deal briefly with these points because each is, in pith and substance, covered under the issues above.
The burden and standard of proof
198. The burden and standard of proof was dealt with fully in Phenytoin: see paragraphs [110] - [117] (on the duty of the CMA to conduct a “full” investigation) and [128]-[140] (on the margin of appreciation of the CMA and the CAT). The Court made the point that whilst the burden of proof to establish an abuse (whether before the CMA or the CAT) is relatively high because of the quasi-criminal nature of the jurisdiction, there was nonetheless an evidential burden upon an investigated undertaking and that the response of the CMA (or CAT) would be proportionate to the evidence placed before it. The CJEU in Generics (UK) (ibid) (see paragraphs [118] above) made clear that the evidential burden on an undertaking seeking to justify conduct that was otherwise abusive is a real, tangible, obligation. In the present case both the CMA and the CAT grappled fully with the applicants’ pricing evidence. The rejection of that evidence cannot be equated with a failure to meet the burden of proof or apply the correct standard of evaluation. For the reasons I have given above the CAT was entitled to reject that evidence upon its intrinsic merits.
The relative evidential value of Cost Plus v alternative benchmarks and comparators.
199. The acceptance of Cost Plus as a test has been acknowledged in jurisprudence for nearly 50 years as providing accurate evidence of pricing in a workably competitive market (see paragraph [77] above). That case law cannot now be gainsaid. Phenytoin made clear that in an appropriate case Cost Plus could be sufficient standing alone to establish excessive pricing but also highlighted the desirability of cross-checking any one piece of evidence (including Cost Plus) against other pieces of available evidence and emphasised that where an investigated undertaking advanced counter evidence the duty of the decision maker was to evaluate that evidence fairly and impartially. In this case the CAT conducted a comprehensive and careful analysis of the CMA reasoning on Cost Plus. There is no appeal against these conclusions. It also compared its conclusions on Cost Plus against a series of comparables: See paragraph [128(vii)] above for a summary. There is no appeal against the conclusions of the CAT on these comparables. In addition, it conducted a detailed analysis of the applicants’ evidence to see whether its own conclusions needed to be moderated. It decided, on the evidence, that they did not. The applicants’ objection to Cost Plus based upon the burden and standard of proof fail on the facts.
Evidence corroborative of Cost Plus
200. The applicants are incorrect in the submission that the CMA and CAT rested their respective conclusions upon Cost Plus alone. The CMA examined a variety of comparator evidence which it concluded was consistent with its analysis of Cost Plus. The CAT considered this evidence and concluded that it was valid and meaningful. The applicants have not performed a side-by-side evaluation of the (unchallenged) comparables and benchmarks relied upon the CAT to support Cost Plus relative to those relied upon by the applicants to challenge Cost Plus. When such an evaluation is performed the main point of difference is that the evidence relied upon by the CAT amounted to actual and current prices for Liothyronine and other drugs, the details of which would have been available to Advanz during the Infringement Period; whereas the comparables and benchmarks relied upon by the applicants are hypothetical and/or concern evidence that would not have been available to Advanz during the Infringement Period.
The applicant benchmarks carry greater probative weight because they reflect convergent pricing
201. In any case where independent sources of evidence converge, they may be said to corroborate each other such that collectively they might weigh more heavily in the evidential scales than the sum of the individual parts. The applicants argue that their evidence is entitled to greater probative weight because it was “convergent”. I can see that in one sense the evidence of how the prices of Teva, Morningside and Advanz responded to each other from 2017 onwards can be said to reflect convergence. Indeed, the new pricing evidence demonstrates a convergent upwards trend. However, convergence by itself is equivocal. A price fixing cartel might generate pricing that is convergent but that evidence hardly reflects effective competition. To the extent that the applicants’ pricing evidence reveals “convergence”, the CAT held that this was explicable by virtue of “soft” and ineffectively competitive market forces. The convergence was not in respect of strands of otherwise independent evidence. This being so convergent pricing patterns post-entry are not explicable only by the forces of workable competition. The evidence the CAT relied upon showed a higher degree of relevant convergence because the Cost Plus figure of £4.94 was much more in line with the prices of comparables charged during the Infringement Period.
Legal certainty
202. The argument that the applicant’s pricing evidence is more legally certain (and hence of greater weight) than that relied upon by the CAT is wrong. The applicant’s central case concerns Cost Plus. As explained above (paragraphs [142]-[145]) the principle of legal certainty favours evidence about Cost Plus and comparables that the dominant undertaking was or could have been aware of, over pricing evidence that the dominant undertaking could not have been aware of at the time of the breach such as EIP, PEP or MFP. Moreover, as set out above at paragraph [200] above, the comparables relied upon by the CAT are more legally certain, in the sense in which that concept is understood in case law, than those relied upon by the applicants.
O. Issue IX: Penalties for specific deterrence: The approach of the CAT when applying the Statutory Guidance to the calculation of penalties designed to create a specific deterrent to future repetition or infringement by the undertaking in question.
The issue
203. Issue IX concerns the application of the CMA for permission to appeal in relation to the judgment of the CAT on penalties, and, if permission is granted for the appeal to be allowed. The issue concerns the interpretation and application of the CMA guidance on penalties as it applied to Cinven. Before coming into effect draft guidance is consulted upon and requires the approval of the Secretary of State pursuant to section 38(4) CA 1998. When setting the amount of a penalty the CMA is required to have regard to guidance in force at the time of the setting of the penalty (section 38(8) CA1998). An obligation upon the CAT to have regard to the guidance was inserted into section 38 CA 1998 by the Enterprise and Regulatory Reform Act 2013. The most up-to-date version at the time of the CMA Decision was dated 18th April 2018 and was entitled “CMA’s guidance as to the appropriate amount of a penalty” (“the Guidance”). This sets out a 6 step approach to the setting of a penalty.
204. The CMA seeks permission to appeal the Judgment where the CAT decided, under Step 4, not to accept an incremental sum of £14.8m which had been included in the CMA’s determination of the overall penalty as necessary to achieve “specific deterrence”. The CMA argues that the CAT misconstrued and misapplied the Guidance by failing to address whether the increment was necessary and/or proportionate when set against the total turnover of the Cinven undertaking as a whole whether in the relevant product market or otherwise; and, in imposing an unwarranted obligation upon the CMA to produce additional specific, evidence based, reasons to support the contention that Cinven might not be incentivised to comply with the law in the future absent the increment in dispute.
General and specific deterrence - the statutory framework and the Guidance
205. The application turns upon the role that deterrence plays in the setting of penalties. Through section 36(7A) CA 1998 Parliament has attached particular importance to deterrence. There are two types of deterrence: general and specific. The section provides that in fixing a penalty the CMA must have regard to the seriousness of the infringement concerned and the “desirability of deterring both the undertaking on whom the penalty is imposed and others from … engaging in conduct which infringes the Chapter II prohibition”.
206. Paragraphs [1.3] and [1.4] of the Guidance explains:
“Policy objectives
1.3 Consistent with section 36(7A) of the CA98, the twin objectives of the CMA's policy on financial penalties are:
• to impose penalties on infringing undertakings which reflect the seriousness of the infringement; and
• to ensure that the threat of penalties will deter both the infringing undertakings and other undertakings that may be considering anti-competitive activities from engaging in them.
The CMA has a discretion to impose financial penalties and intends, where appropriate, to impose financial penalties which are severe, in particular in respect of agreements between undertakings which fix prices or share markets, other cartel activities and serious abuses of a dominant position. The CMA considers that these are among the most serious infringements of competition law.
1.4 There are two aspects to the deterrence objective. First, there is a need to deter the undertakings which are subject to the decision from engaging in future anti-competitive activity (often referred to as 'specific deterrence'). Second, there is a need to deter undertakings at large which might be considering activities contrary to any of Article 101, Article 102, the Chapter I or Chapter II prohibitions from breaching the law (often referred to as 'general deterrence').”
207. The Guidance mandates a 6 step approach to the computation of a penalty. The imperative of general deterrence is built into Step 1 (on seriousness) and that of specific deterrence into Step 4 (on deterrence and proportionality). These steps are summarised in the Guidance at paragraph [2.1] as follows:
“Method of calculation
2.1 A financial penalty imposed by the CMA under section 36 of the CA98 will be calculated following a six-step approach:
• Calculation of the starting point having regard to the seriousness of the infringement and the relevant turnover of the undertaking.
• Adjustment for duration.
• Adjustment for aggravating or mitigating factors.
• Adjustment for specific deterrence and proportionality.
• Adjustment if the maximum penalty of 10% of the worldwide turnover of the undertaking is exceeded and to avoid double jeopardy.
• Adjustment for leniency, settlement discounts and/or approval of a voluntary redress scheme.”
208. General deterrence is concerned with ensuring that other undertakings are dissuaded from engaging in unlawful conduct in particular of a type engaged in by the undertaking in question. General deterrence is considered under Step 1. At this stage the CMA is required to make an overall assessment of seriousness. Paragraph [2.9] provides: “Finally, the CMA will consider whether the starting point for a particular infringement is sufficient for the purpose of general deterrence. In particular the CMA will consider the need to deter other undertakings, whether in the same market or more broadly, from engaging in the same or similar conduct.”
209. Specific deterrence serves a different policy objective. It is covered in Step 4. This comes into play after the CMA has considered seriousness, duration, aggravating and mitigating factors. The CMA is required to look at the undertaking as a whole taking account of a range of factors outside the product market in which it was dominant. The Guidance is as follows:
“Step 4 - adjustment for specific deterrence and proportionality
2.20 In considering whether any adjustments should be made at this step for specific deterrence or proportionality, the CMA will consider appropriate indicators of the undertaking's size and financial position at the time the penalty is being imposed. The CMA may have regard to indicators - including, where they are available, total turnover, profitability (including profits after tax), net assets and dividends, liquidity and industry margins - as well as any other relevant circumstances of the case. The CMA will generally consider three year averages for profits and turnover. The CMA may also consider indicators of size and financial position from the time of the infringement.
2.21 The penalty figure reached after steps 1 to 3 may be increased to ensure that the penalty to be imposed on the undertaking will deter it from breaching competition law in the future, given its specific size and financial position and any other relevant circumstances of the case. Such an increase will generally be limited to situations in which an undertaking has a significant proportion of its turnover outside the relevant market or where the CMA has evidence that the infringing undertaking has made or is likely to make an economic or financial benefit from the infringement that is above the level of penalty reached at the end of step 3. Where relevant, the CMA's estimate would account for any gain which might accrue to the undertaking in other product or geographic markets as well as the 'relevant' market under consideration. The assessment of the need to adjust the penalty will be made on a case-by-case basis for each individual infringing undertaking.
2.22 In addition, there might be exceptional cases where an undertaking's relevant turnover is very low or zero with the result that the figure at the end of step 3 would be very low or zero. In such cases, the CMA would expect to make more significant adjustments, both for general and specific deterrence, at this step. Such an approach may also be appropriate where the relevant turnover did not accurately reflect the scale of an undertaking's involvement in the infringement or the likely harm to competition. This might be the case, for example, in relation to bid-rigging cases or where an undertaking's turnover in the last business year before the infringement ended was unusually low.
2.23 In considering the appropriate level of uplift for specific deterrence, the CMA will ensure that the uplift does not result in a penalty that is disproportionate or excessive having regard to the undertaking's size and financial position and the nature of the infringement.”
210. The Step 4 increment takes account of the level of penalty at Step 3 and may be increased or decreased accordingly to take account of overall proportionality:
“2.24 At this step, the CMA will assess whether, in its view, the overall penalty proposed is appropriate in the round. Where necessary, the penalty reached at the end of steps 1 to 3 may be decreased to ensure that the level of penalty is not disproportionate or excessive. In carrying out this assessment of whether a penalty is proportionate, the CMA will have regard to the undertaking's size and financial position, the nature of the infringement, the role of the undertaking in the infringement and the impact of the undertaking's infringing activity on competition.”
211. Being “Guidance” there is no statutory duty upon the CMA (or the CAT) to follow its strictures slavishly in all cases. However, given the obvious significance that Parliament has attached to the Guidance, including making it subject to Secretary of State approval, general principles of good administration dictate that any departure must be objectively justified and reasoned: see for a like conclusion Argos Ltd. and Littlewoods Ltd. v. OFT [2006] EWCA Civ 1318 at paragraphs [162]-[163].
The CMA decision on Step 4
212. The CMA’s application focuses upon the approach of the CAT in applying Step 4 of the Guidance. The CMA complains that the CAT erred in its analysis of whether an increment for specific deterrence was justified over and above the conclusion arrived at under Step 3 of the Guidance.
213. Under Step 3, adjustment for aggravating and mitigating factors, the CMA took account of the involvement of directors and senior management in the infringement. The CMA rejected, as mitigation: that there was genuine uncertainty as to whether the pricing conduct was unlawful; that the pricing had been approved of by the DoH or, at least, not objected to; that the infringement was not intentional; that there had been full cooperation with the CMA; and that all parties had taken adequate steps with a view to ensuring and promoting competition or compliance. No part of the analysis under Step 3 involved a consideration of specific deterrence.
214. In relation to Step 4 the CMA distinguished between specific and general deterrence: Decision paragraph [7.123]. In paragraph [7.124] the CMA, citing relevant EU case law, stated that the object of pursuing a specific deterrent effect was “... essentially to control, in the future, the conduct of the economic entities to which the decision is addressed. Such an effect must necessarily be produced on the undertaking in the state in which it exists at the time when that decision is adopted”.
215. In Decision paragraphs [7.143]-[7.179] the CMA applied the principle of specific deterrence to each of the entities responsible for the abuse during the Infringement Period and considered whether, in its view, the imposition of an increment to reflect the need for specific deterrence was proportionate.
216. The CMA imposed a penalty of £51.9 million upon Cinven which included a component of £14.8m to address specific deterrence. The CMA took into account four matters:
(i) The Step 3 penalty of £37.1 million did not materially exceed the minimum financial benefit (“MFB”) of £34.1 million earned by Advanz from the abuse during the period when Advanz was controlled by Cinven. A penalty exceeding the MFB by only 8.6% was insufficiently high to have a deterrent effect upon an undertaking the size and strength of Cinven (Decision paragraph [7.150]).
(ii) The Step 3 penalty was just 0.3% of Cinven’s average worldwide relevant turnover of £14.4 billion in the last three financial years before the Decision (Decision Table 7.6). A larger penalty was therefore required to achieve the required deterrent effect and to command the appropriate degree of attention from top-level management (Decision paragraphs [7.151-152]).
(iii) Cinven generated more than 99% of its total turnover outside the relevant market. A significant upwards adjustment to the Step 3 penalty would ensure the penalty had a more material financial impact on Cinven in the context of its overall business (Decision paragraphs [7.153] - [7.156]).
(iv) Finally, the serious harm the infringement caused to the NHS and ultimately patients warranted an additional uplift over the Step 3 total (Decision paragraph [7.159]).
The CAT Judgment on Step 4
217. The CAT accepted that save in respect of the uplift for specific deterrence the penalties imposed were justified. The CAT reduced the penalty upon Cinven to £37.1 million removing the component for specific deterrence. The reasoning of the CAT on the approach of the CMA to Step 4 is at Judgment paragraphs [480]-[487] and can be summarised as follows:
(i) The calculation of a deterrence uplift by reference to the financial benefit accruing to Advanz as a result of its infringing conduct was “unimpeachable” (Judgment paragraph [480]).
(ii) The benefit flowing to the entities liable for the infringement was below the penalty reached under Step 3, but it was legitimate to conclude that a further uplift was required (Judgment paragraph [480]).
(iii) The gain was easy to compute and would not complicate or render unfair any subsequent damages action (Judgment paragraph [483]).
(iv) It was right not to set off profits from an increased volume of sales at a lower price in calculating the gain (Judgment paragraph [484]).
(v) The CMA correctly addressed relevant indicators of the entities financial position including profits after tax, net assets, the level of dividends and industry margins (Judgment paragraph [485]).
(vi) The argument of Cinven that the CMA’s consideration of its turnover outside the relevant market discriminated unlawfully against private equity investors (Judgment paragraphs [486]-[487]) was rightly rejected.
218. Where the CAT disagreed with the CMA was in its conclusion that an uplift to the Step 3 penalty was necessary to deter Cinven from breaching competition law in the future. The reasoning on this is set out in Judgment paragraphs [488]-[494]. The principal reasons are set out in paragraphs [488]-[492] and secondary, supporting, reasons are set out in paragraphs [492]–[494]. The primary reasoning can be summarised as follows:
(i) The Step 3 penalty of £37.1m was 8.6% above the MFB to Cinven of 34.1m.
(ii) This sufficed to deprive Cinven of any commercial gain from the infringement.
(iii) The 8.6% increment above the MFB was “relatively small in percentage terms” but it was not “immaterial”.
(iv) It was a reasonable assumption that the Cinven management would be “mindful” of “reputational damage” resulting from the Decision which would act as a further deterrent against future infringements.
(v) The CMA had failed to identify any “specific reason” for believing that absent a deterrent uplift there was a risk of further breaches of competition law by Cinven or unlawful conduct which might escape detection or enforcement.
219. Paragraphs [493]-[494] set out secondary or corroborative considerations attracting “some weight” in the analysis. This concerned the existence of powers under section 262 NHS Act 2006, as amended by the Health Service Medical Supplies (Costs) Act 2017 which conferred additional powers of regulation upon generic pharmaceutical suppliers.
The CMA’s proposed grounds of appeal
220. The CMA argues that, in respect of both the principal and secondary reasoning, the CAT erred in its interpretation and application of the objective of specific deterrence under Step 4 of the Guidance. It is said that the CAT (i) failed to take into account relevant considerations and (ii) took into account irrelevant considerations.
Failure to address relevant considerations
221. The CMA argues that, in accordance with the Guidelines, the CAT failed to address (a) the size and financial strength of the Cinven undertaking; (b) the fact that it generated a significant proportion of its turnover outside the relevant market; and (c), the conservative approach taken by the CMA to the calculation of the MFB.
222. The first consideration in determining whether to adjust the Step 3 penalty is the size and financial position of the undertaking in question: Guidance paragraph [2.20]. It was addressed by the CMA in the Decision at paragraphs [7.151]-[7.152]. Where a penalty represented a small percentage of the total turnover of an infringing undertaking the impact of that penalty upon the undertaking as a whole would be limited. In this case the Step 3 penalty was very small. It was c.0.26% of Cinven's worldwide turnover in 2019. The CMA argues that “… on any view such a penalty would be insufficient to ensure that Cinven be given the necessary incentives to comply with competition law in the future.” The CAT erred because it failed to mention this as a relevant and important consideration.
223. The second consideration under Guidance paragraph [2.21] takes into account whether a significant proportion of the turnover of an undertaking is generated outside the relevant market. If this is ignored there is a risk that a figure arrived at after Step 3 is insufficient to exert a real impact upon the undertaking in the context of its overall business. The CMA took this consideration into account in Decision paragraphs [7.153] - [7.156]. Again this is not referred to in the Judgment.
224. The third complaint flows from the reasoning in Judgment paragraph [489] which, it is said, reflects a misunderstanding of the role played by the policy imperative of neutralising commercial gain through a suitably high level of penalty. Guidance paragraph [2.21] provides that an upward adjustment at Step 4 is generally limited to two situations one of which is where the CMA has evidence that the infringing undertaking has made or is likely to make in the future an economic or financial benefit from the infringement that exceeds the Step 3 penalty. In Decision paragraph [7.144] the CMA explained:
“It is an important part of effective deterrence that an undertaking should not be in a position to earn a profit from infringing competition law even after paying a penalty in respect of that infringement. Nor is it sufficient for any penalty to only neutralise an infringing undertaking’s direct financial gains resulting from an infringement. If the penalty imposed on an undertaking for a competition law infringement only neutralises the gains made (i.e. puts the undertaking in the same position as it would have been absent the infringement) there is little economic incentive for the undertaking not to infringe competition law again: at most, it would risk losing its gains if it was caught and sanctioned.”
225. In the Decision the CMA calculated the MFB as follows. First, it calculated the difference between (1) the lowest price charged for Liothyronine Tablets found to have been excessive (£20.48) and (2) Advanz’s ASP during the period of each entity’s ownership of Advanz. Secondly, from this it calculated the gain from the infringement during the period Advanz was controlled by Cinven (£34.1 million). Thirdly, it calculated the extent to which the Step 3 penalty of £37.1 million was above the gain, which it calculated as a percentage (8.6%). Fourthly it calculated the gain as a percentage of Cinven’s worldwide turnover (0.02%). The CAT (Judgment paragraph [480]) upheld the calculation made by the CMA of the MFB flowing from the breach. It held, however, at paragraph [489], that the Step 3 penalty was sufficient to deprive Cinven of any commercial benefit. They were “relatively small in percentage terms” but they were, nonetheless, not “immaterial”.
226. The CAT erred because the gain calculated by the CMA did not represent the true gain from the abuse. This was because the CMA used conservative assumptions in calculating the MFB (for a summary see paragraphs [43]-[47] above). First the CMA, for reasons of prioritisation, focused only upon prices above £20.48 per pack and not Cost Plus or some figure between Cost Plus and £20.48: Decision paragraph [5.105]. Secondly, the MFB did not take into account financial benefit from the infringement arising following its termination in July 2017 (Decision paragraph [7.134]) even though the abuse took a considerable period of time to unwind and even though prices a number of years post-entry remained contaminated by the earlier unlawful conduct. The CMA explained (Decision paragraph [7.149]) that these assumptions meant that the true MFB was above the level of the unadjusted penalty set out under Step 3.
227. Pulling threads together the CMA argues:
“Taking these three considerations together, the CMA’s overriding criticism is that the CAT has failed to take into account factors identified in Step 4 of the Guidance, which it must have regard to when setting a penalty: s. 38(8) CA 1998… These factors are so obviously material to a decision fixing a penalty that anything short of direct consideration of them by the CMA or the Tribunal would not be in accordance with the purpose of s. 36(7A)(b) CA 1998.”
Taking into account irrelevant considerations
228. The second complaint is that the CAT took into account irrelevant considerations: (i) a perceived requirement on the part of the CMA to adduce evidence or reasons for believing that, absent such an uplift, there was a risk of further breaches of competition law by the undertaking (Judgment paragraph [491]); and (ii), the existence of powers under section 262 NHS Act 2006, as amended by the Health Service Medical Supplies (Costs) Act 2017 (Judgment paragraph [494]).
229. In relation to the first complaint, it is argued that neither the CA 1998, the Guidance, nor any binding authority, established that the CMA was required to evidence a risk of future breach by a specific undertaking. Such a task was excessively difficult, if not impossible, to demonstrate. To require the CMA to adduce evidence of such a risk was an error of principle. The factors identified in the Guidance paragraphs [2.20] and [2.21] were sufficient to justify an uplift for specific deterrence irrespective of additional evidence of a propensity to re-offend. The approach of the CAT undermined the objective of the statutory policy behind deterrence in penalty setting.
230. As to the second complaint (the existence of powers on the part of the medical authorities to regulate prices), the response of the CMA, which the CAT did not address, is set out in Annex 7 to the Decision at paragraphs [7.66]-[7.70]. The CAT considered that notwithstanding these points the existence of the powers should be given “some weight”, though without explanation. It does not quantify “some”. The CMA argues that the statutory powers are not yet in force. Whilst the DHSC had publicly committed to consulting upon its methodology for deployment it had not yet done so as of the date of the CAT appeal. It was far from certain how effective any powers would be in practice. The powers extended only to the prices of individual unbranded generic medicines whereas specific deterrence covered all of an infringing undertakings economic activity which in the case of Cinven operated in financial services, media and healthcare. The possibility of future regulation of individual generic drugs was irrelevant when assessing the need for a deterrent uplift in the present case.
The submissions of Cinven
231. Cinven argues that the CAT did not err. It was not bound by the views of the CMA and was required to exercise independent judgement. Its application of the Guidance was impeccable but if it did depart from the Guidance it was entitled to do so. This Court should be loath to interfere in a judgment call of the CAT which had regard to all the facts. Although the statutory jurisdiction of the Court on an appeal is broad (see paragraph [28] above) the Court should interfere only rarely where it was plain the CAT has gone badly wrong, which was not so in this case: See Interclass Ltd. v. OFT [2012] EWCA Civ 1056 per Patten LJ at paragraph [59]. Cinven also argues that the reduction in penalty was justified since otherwise there would be unequal treatment and/or unfairness arising from the fact that the CMA has not appealed the reduction in fine imposed upon Hg. In the Guidance (footnote 17) it is recognised that the CMA has a duty of equal treatment when imposing penalties upon the different parties to an infringement. The CMA cites the articulation of the principle by the General Court of the EU which is in the following terms: “… the fact nonetheless remains that [the decision maker] must comply with the principle of equal treatment, according to which it is prohibited to treat similar situations differently and different situations in the same way, unless such treatment is objectively justified”: See case T-236/01 Tokai Carbon Co limited and others v Commission [2004] ECR II-1181 at paragraph [219].
Analysis
232. I start with general observations about the jurisdiction of this Court. The jurisdiction under section 49(1) CA 1998 is broader than in relation to appeals on substantive (non-penalty) issues. In Phenytoin this Court explained that there was a requirement for a rigorous merits jurisdiction before the CAT because the regime for competition law enforcement was treated as quasi-criminal: Phenytoin paragraph [140] cited at paragraph [27] above. It is for this reason that in penalty cases the Court of Appeal also had a jurisdiction broader than that applicable to substantive merits. In Interclass (ibid), which predated Phenytoin, this Court observed that even though the jurisdiction was broad the Court would exercise a degree of reticence:
“This Court’s jurisdiction is governed by s.49(1) of the 1998 Act and is not limited (in relation to penalty) to errors of law by the CAT. But in a case where there is no real challenge to the primary findings of fact this Court is limited to a review of the penalties based on the material before the CAT. Given the specialist nature of the tribunal and its obvious expertise in these matters, an appeal against penalty is unlikely to be successful unless it can be shown either that the CAT erred in principle (which can include a failure to take relevant matters into account) or that, looked at overall, the penalties imposed were clearly disproportionate or discriminatory so as to be unjustifiable by any of the matters which the CAT either did or should have taken into account.”
233. I agree with this note of caution, save for the use of the word “clearly” in relation to proportionality and discrimination which I do not think adds anything to the analysis. Either an exercise of discretion is disproportionate or discriminatory, or it is not. I would eschew a requirement that a finding of disproportionality or discrimination must have some added ingredient, before this Court will intervene. Against this background are the Guidelines. As guidance they do not bind but, as already observed, they import with them significant weight (see paragraphs [211] above) and the CMA and the CAT should not depart from them without objective reason and it should give a proper explanation.
234. In the present case the CAT purported to apply the Guidance. This is evident from the structure of the CAT’s analysis and the absence of any statement that it was seeking to apply independent, self-standing, reasoning. The CAT is entitled to form its own views on each of the matters set out in the Guidance. It is not, therefore a valid ground of criticism that the CAT did not adopt the reasoning of the CMA and I reject the suggestion of the CMA made in oral submission that simply because the CMA exercised its discretion in a manner which might to be said to be reasonable, that prevented the CAT exercising the discretion differently.
235. The crux of the issue is whether the CAT failed properly to construe and/or apply the Guidance. On this I agree with the submissions of the CMA summarised above. I set out my conclusions relatively briefly.
236. First, in Judgment paragraph [489] the CAT addressed whether the conclusion arrived at under Step 3 was sufficient to address the requirement for an incremental sum to be added under Step 4. The CAT compared the penalty imposed upon Cinven with the MFB and calculated that it was 8.6% higher. Such a percentage was “relatively small”, but it was not “immaterial”. And for this reason, it was not “necessary” to increase the increment for specific deterrence (Judgment paragraph [492]). In my judgement the CAT erred. Step 4 requires a different calculation to be undertaken. Under Step 4 the Step 3 level penalty (i.e. absent the increment for specific deterrence) should be measured against the total, global, turnover of the undertaking, not the different exercise of comparing the Step 3 level penalty against the MFB. The imposition of a fine which is a “relatively small” percentage above MFB does not indicate whether the undertaking will be incentivised to engage in further infringements. In this case a Step 3 penalty measured against global turnover shows that the Step 3 penalty is de minimis, which is the opposite conclusion arrived at by the CAT. Had the CAT correctly carried out this calculation I do not think that it would have arrived at the conclusion it did, quite irrespective of the other reasons it gave for not increasing the penalty beyond the Step 3 level. I turn to those other reasons.
237. Secondly, in Judgment paragraph [490] the CAT considered that it could reasonably assume that the Cinven management would be “mindful” of “reputational damage” which would flow from the Decision, and this would act as a deterrent against future infringements. The CAT does not explain why this is a reasonable assumption to make from the mere fact of the Decision. It might be that it was referring to the reputational fallout from the imposition of penalties pursuant the Decision, and not the Decision per se. But even construing the Judgment in this way any penalty, including one fixed at Step 3 levels, is capable of inflicting reputational damage. Whether this acts as a deterrent against future infringements is a different question addressed by applying the Step 4 test in the Guidance, including a comparison of the Step 3 level penalty against the global financial power of the undertaking in question. An inference that an undertaking might be mindful of reputational damage is not an answer to the question - might it nonetheless be incentivised to commit further offences?
238. Thirdly, in Judgment paragraph [491] the CAT says that the CMA did not point to any specific reason for believing that, absent a deterrent uplift, there was a risk of further infringements or that Cinven might engage in future unlawful conduct that was beyond detection or enforcement. With respect, the CMA did identify such a reason, namely the de minimis nature of the Step 3 level penalty relative to the global turnover of the undertaking. Under the Guidance, and consistent with the overarching statutory purpose, this is treated as an indication that there is a risk of recidivism.
239. Fourthly, a more structured approach to the application of Step 4 does not mean that the CAT is precluded from nonetheless limiting a penalty to Step 3 levels. It is entitled under the Guidance to do this by reference to the overarching test of proportionality under Step 4. If, for example, the Step 3 level penalty had been, say, 100% above the MFB, the CAT might then have addressed, quite properly, whether it would be disproportionate to impose an additional increment for specific deterrence. But the CAT never asked itself, in the circumstances of this case, whether it was disproportionate to impose an increment for specific deterrence amounting to a tiny fraction of Cinven’s global financial turnover when the Step 3 level penalty was only 8.6% above the MFB.
240. Fifthly, in relation to the argument about new legislation this is not a big point. The CAT simply stated that it would attribute “some weight” to the existence of the statutory powers of intervention. I agree with the analysis of the CMA set out at paragraph [230] above. As matters presently stand the ability of regulators to exercise new powers to curb excessive pricing is limited. Whether that changes in the future is irrelevant for the purpose of this case.
241. Finally, in relation to the argument of Cinven about discrimination and equal treatment, in my judgment, there is no unequal treatment. Hg settled its case with the CMA and hence there was no application by it for permission to appeal and there was no cross application by the CMA. There is no suggestion that the settlement of the litigation was in any way improper, or outwith the power of the CMA to conclude. I do not consider that Hg and Cinven are in an objectively comparable position and/or that the CMA treatment of Cinven relative to Hg is not objectively justified.
P. Disposition
The approach to take
242. I turn to conclusions on the applications. The first task is to decide whether to grant permission to appeal. The second task is to consider, in relation to any ground where permission is granted, whether to allow the appeal. The jurisdiction of this Court on the substantive matters (Issues I-VIII) is limited to points of law. A dispute which focuses upon matters of fact can, properly analysed, amount to an objection of law. This is the case, for instance, where the complaint is that: the decision maker acted outside the bounds that might be expected of a reasonable or rational decision maker; the decision maker took into account an irrelevant factual matter; or, the decision maker failed to take into account a relevant factual matter. In Cérélia Group Holdings and others v CMA [2024] EWCA Civ 352 at paragraphs [27]-[41], in the context of a judicial review, the Court summarised existing case law on the circumstances where, even in a judicial review, disputes over fact can amount to justiciable grounds upon the basis that the decision maker made fact findings which were beyond the bounds of rationality or reasonableness. In a merits appeal such a challenge might give rise to an issue of law.
243. The fact that a dispute centres around facts or evidence does not therefore mean that it is incapable of amounting to an issue of law. There is though often a thin dividing line between a properly arguable issue of law and one that is simply a camouflaged challenge to findings of fact. Separating these out can create difficulties at the paper PTA stage. As was pointed out in Airwave Solutions Limited and others v CMA [2025] EWCA Civ 54 at paragraph [88] it is not always easy, in competition cases, without having a detailed understanding of often highly complex facts, to know at the PTA stage whether a proposed ground which focuses upon facts but which is articulated as a ground of law is, in truth, properly so categorised.
244. At the PTA stage if a proposed ground articulated as an issue of law meets the test for an appeal, namely that it has a real (and not fanciful) prospect of success, then the case proceeds as a fully-fledged appeal even though, in the final judgment, the ground might be rejected upon the basis that, properly analysed, it is really an issue of fact and nothing else. In such a case the Court does not backtrack and revoke permission to appeal; it simply rejects the appeal.
245. Here there has been a rolled up hearing during which the Court has received comprehensive oral and written submissions on all issues. In such a case it is not open to the Court, jurisdictionally, to grant permission to appeal to an argument that is clothed as an issue of law but which the Court concludes is, in substance, an objection to a finding of fact. The inevitable outcome in such a case is that permission to appeal must be refused. It is not that permission be granted but the appeal then dismissed.
Standing back
246. There are four facts and matters which are incontrovertible before this Court.
247. First, the CAT endorsed the reasoning and conclusions of the CMA on the calculation of the Cost Plus figure (£4.94). There is no challenge to the calculations or analysis of the CAT on that issue.
248. Secondly, the CAT held that evidence of comparables, including for Liothyronine, relied upon by the CMA as benchmarks for its conclusion on Cost Plus were “validly made” and “meaningful”. These comparables related to prices applied during the Infringement Period and they supported the findings of the CMA on Cost Plus: Judgment paragraph [277]. There is no challenge to the conclusion that the comparables were valid and meaningful nor any submission that they attracted lower evidential value than prices for Liothyronine after the Infringement Period.
249. Thirdly, before the CAT no attempt was made to justify, economically, the higher prices charged towards the latter part of the Infringement Period (up to £247) upon the basis that they reflected prices generated under conditions of workable competition. The applicants sought only to justify prices materially below £100 as non-abusive. The extent of the effort to justify the higher prices was (and remains) entirely legal (e.g. acquiescence).
250. Fourthly, a sense check on the applicants’ argument that their test is better for consumers than Cost Plus, is that the prices the applicants contend are lawful are about 10 times higher than those based upon Cost Plus, which was where (roughly) prices stood in 2007, before Advanz embarked upon its price optimisation strategy. Had Advanz maintained prices on this latter basis throughout the Infringement Period, and had no entry therefore occurred to stimulate new competition leading to loss of dominance, the NHS (as proxy for the consumer) would have been substantially better off.
251. According to the law as summarised in Phenytoin, abuse can be established with evidence of Cost Plus alone or Cost Plus benchmarked against comparables. That is the position in this case. These are valid methods for determining what a fair price would be in conditions of workable competition: Phenytoin paragraph [97(v)-(vii)].
252. The issue before the Court is hence really quite narrow. It is whether the quality of the evidence of pricing relied upon by the applicants is so superior to that relied upon by the CAT, that it renders the otherwise valid conclusion of the CAT that Cost Plus (benchmarked against current pricing of comparables) is a proper basis for the finding of abuse, wrong in law. In circumstances where the unchallenged findings of the CAT suffice, standing alone, to establish abuse, this is a tall order for the applicants.
Conclusion on applications
253. With this in mind I take the issues arising in sequence.
254. In my judgement issues I-VI on pricing, are, at their core and in their true substance, objections to findings of fact made by the CAT. The CAT addressed itself fully to the applicants’ evidence. It cannot be said that it was overlooked. It was simply rejected after fair evaluation. There can be no appeal against the CAT’s findings on the evidence. As to the applicants’ arguments about the law, for instance that certain measures of pricing amounted to mandatory tests and were dispositive, many of these measures were not supported by the experts and in pith and substance reflect attempts using forensic alchemy to transmogrify disputes over evidence into points of law. For the reasons I have given they are not arguable. It follows that in relation to these arguments permission to appeal must be refused.
255. In relation to issue VII, acquiescence, the CAT rejected the applicant’s case upon the facts. The points of law raised are academic. Permission to appeal is refused.
256. In relation to Issue VIII, burden and standard of proof, the analysis of facts in the judgment of the CAT was careful, comprehensive and to the point. There is no basis upon which it can properly be argued that on the evidence the CAT failed to apply the requisite burden and standard of proof and acted outside the scope of its legitimate discretion to find facts. Permission to appeal is refused.
257. In relation to Issue IX, penalties, the Court has jurisdiction to entertain appeals on both law and fact. In my judgement the CAT erred in its application of the Guidance which it is required in law to take account of. The proposed ground of appeal advanced by the CMA is properly classified as one of law and this Court has jurisdiction to entertain an appeal. For the reasons set out above (paragraphs [203]-[241]) I conclude that the CMA’s submissions meet the standard for the grant of permission and are correct. Permission to appeal must therefore be granted and the appeal allowed. An issue then arises as to whether to remit the issue of penalty to the CAT for reconsideration. This Court has the same powers as the CAT: Section 15(3) Senior Courts Act 1981 considered in Evans v Barclays Bank [2023] EWCA Civ 876 at paragraphs [159]-[163]. In my judgement the appropriate course is for this Court to reinstate the full penalty imposed on Cinven by the CMA in the Decision.
258. I would finally express my gratitude to all Counsel for the conspicuous quality and clarity, and often ingenuity, of their written and oral submissions.
Lord Justice Zacaroli :





