This judgment was handed down at 10.30 am on 27 January 2025 by circulation to the parties' representatives by email and release (in a redacted form) to The National Archives
Introduction. 1
An outline chronology. 1
The evidence. 1
The fact evidence. 1
The expert evidence. 1
The Timing Issue. 1
The processes in issue. 1
Dr Hill's original case as to the conception and communication of the inventions. 1
November 2007 - January 2008. 1
May - mid October 2008. 1
Late October 2008 - January 2009. 1
The inventorship dispute. 1
Overall assessment. 1
The Contract Issue. 1
The law.. 1
The Service Agreement. 1
The Information Memorandum.. 1
The factual matrix. 1
Assessment. 1
Estoppel 1
Limb 1. 1
Limbs 2 and 3. 1
Other issues. 1
Limitation. 1
Unjust enrichment. 1
Circuity of actions. 1
Redactions. 1
The Deputy Judge:
1. In these proceedings the Claimant ("Dr Hill") seeks various forms of relief against the Defendants (collectively "Touchlight") arising from her claim to be jointly entitled to the invention(s) of certain patents and patent applications stemming from the international patent application filed by the First Defendant ("TGL") on 1 February 2010 as PCT/GB2010/000165 ("the PCT application") and claiming priority from an application filed by TGL on 30 January 2009 ("the priority application").
2. The patents and patent applications in issue in these proceedings ("the Patents") are (i) European Patents (UK) nos. 2391731, 2612925 and 3150722 ("the EP(UK)s") which are now registered in the name of the Second Defendant ("TIP"), (ii) US patent application no. US 2022 0372565 A1 in the name of TIP ("the US application") and (iii) Chinese patent application no. CN104911177A and the corresponding Hong Kong patent application no. HK1215045 in the name of TGL. During the course of these proceedings, Dr Hill applied to extend their scope to encompass further patents, but that application was withdrawn in circumstances explained in the judgment of Joanna Smith J of 10 July 2024: [2024] EWHC 1913 (Pat).
3. It is common ground that the Patents disclose a process for making so-called "doggybone" DNA (or dbDNA) which has been referred to in these proceedings both as "the Close-Ended Process" and "the Direct-Acting Protelomerase Process". While I think the latter term better summarises the key aspect of the process, I shall use the former term because it was used more frequently during the trial. However, it is important to appreciate that neither term was used by the parties at the time of the relevant events. It is also common ground that the Patents disclose a version of the Close-Ended Process in which the starting material is itself doggybone DNA, referred to as "the dbDNA Template Process", though there is a dispute about whether that amounts to a separate concept.
4. Dr Hill's case is that she devised the Close-Ended Process and the dbDNA Template Process before she was employed by TGL as its Chief Scientific Officer ("CSO") under a service agreement which took effect in early September 2008 ("the Service Agreement"). She accepts that the Service Agreement assigned to TGL some of her rights in that invention (or those inventions), but contends that the rights assigned were limited to the processes operated under fully thermophilic conditions (i.e. conditions suitable for enzymes or other proteins derived from thermophilic organisms, in practice above about 45oC).
5. Accordingly, Dr Hill contends, she is jointly entitled to the invention(s) of the Patents and seeks various forms of relief to give effect to that entitlement, including her registration as a proprietor of the Patents and the grant of a retrospective exclusive licence to her in respect of the operation of the processes under mesophilic conditions (i.e. temperatures between about 20oC and 45oC). On that premise, she contends that Touchlight have infringed her rights by operating the processes under mesophilic conditions in the UK and by granting licences to third parties to operate the processes, and seeks financial relief accordingly. She also alleges that TGL and TIP have been unjustly enriched at her expense as a result of granting licences to third parties and seeks restitution.
6. Touchlight's case is that the Close-Ended Process and the dbDNA Template Process were devised after the Service Agreement took effect. In any event, Touchlight says, the Service Agreement would have assigned the invention(s) to TGL if they had been devised before the Service Agreement took effect.
7. In addition, Touchlight (i) contends that Dr Hill's conduct and Touchlight's reliance thereon gave rise to an estoppel (whether by convention, acquiescence, representation or promise) preventing her from asserting any such rights as she might otherwise have had in the invention(s); (ii) pleads a limitation defence; and (iii) contends that Dr Hill's claims for financial relief fail for circuity of actions, either because such relief would amount to unjust enrichment of Dr Hill at Touchlight's expense, or on the basis of a breach of her duties as a director of TGL (claims which it advances by way of counterclaim).
8. The first issue to be determined, therefore, is whether the Close-Ended Process and the dbDNA Template Process were devised before or after the Service Agreement took effect in early September 2008 ("the Timing Issue"). If that is determined against Dr Hill, her claim fails. If it is determined in her favour, then it is necessary to decide whether the Service Agreement was effective to transfer to TGL all her rights in the invention(s) or only those rights in so far as they related to fully thermophilic processes ("the Contract Issue"); if the former, then again her claim fails. If Dr Hill succeeds on both those issues, then it is necessary to consider Touchlight's estoppel case. If that fails, then it is necessary to consider Touchlight's limitation defence and the relief claimed by Dr Hill, including her claim to financial relief / restitution, and Touchlight's arguments about circuity of actions and its counterclaim.
9. At trial, Mr Cuddigan KC made the oral submissions on behalf of Dr Hill and cross-examined Touchlight's fact witnesses, while Ms Pickard cross-examined Touchlight's technical expert. For Touchlight, Mr Speck KC cross-examined Dr Hill's witnesses and made oral submissions on the Timing Issue and limitation, while Ms McKechnie made the oral submissions on the Contract Issue and estoppel. I was pleased to see junior counsel being given the opportunity to conduct significant parts of the trial. I thank all counsel, and the solicitors on both sides, for their work at trial, in the lead up to trial, and indeed after trial in compiling materials which I had requested.
10. The Timing Issue requires focus on documents and evidence relating to the question of whether the Close-Ended Process and the dbDNA Template Process were conceived before or after the Service Agreement came into effect in early September 2008; those materials principally span the period from October 2007 to May 2009. The Contract Issue requires consideration of the factual matrix of the Service Agreement, which was entered into on 8 July 2008; the relevant materials are all before that date but are not limited to those which are important to the Timing Issue. The issue of estoppel depends on events and documents in the period leading up to the filing of the priority application in January 2009 and thereafter, including in particular events in the period 2009 to 2012. When considering each issue I have sought to focus on the materials relevant to the issue in question, but that means that it may be difficult to appreciate the whole chronology of events. It may therefore be helpful to set out an outline chronology against which the more detailed treatment of the issues can be read.
11. From November 1998 until April 2006 Dr Hill held a post-doctoral position at Royal Holloway University London ("RHUL"). While she was there she conducted work which formed the basis for ideas on in vitro production of vector-free DNA expression cassettes for potential application as DNA vaccines or in gene therapy, which included the idea of conducting amplification under thermophilic conditions. Another of her ideas related to using thermophilic bacteria to deliver DNA "warheads" to pathogenic agents (this is the basis of what became known as the ThermoLethal Vectors project).
12. A quite distinct area of interest for Dr Hill was "Nature's Code". This was theoretical work which crossed the disciplines of biology and physics which she was conducting together with the theoretical physicist Dr Peter Rowlands. RHUL gave Dr Hill an honorary position from April 2006 to July 2007 so that she could have an academic address from which publish her work on Nature's Code. Dr Hill and Dr Rowlands presented a paper on Nature's Code at the CASYS conference in Liège in August 2007, winning its prize for best paper.
13. In late 2006 Dr Hill was interested in the idea of founding a biotechnology company to exploit some of her ideas, including those relating to vector-free DNA vaccines, and in early 2007 she approached Mr Jonathan Ohlson, a businessman. Nothing came of that initial contact but in May 2007 she approached Mr Ohlson again. As will be seen below, Dr Hill's pleaded case was that by that time she had conceived of both the Close-Ended Process and the dbDNA Template Process.
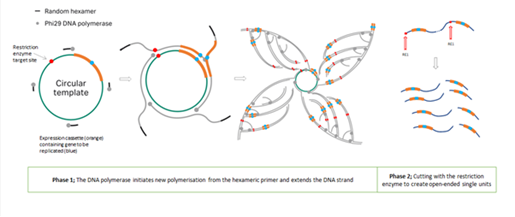
14. In September 2007 Dr Hill proposed Dr Neil Porter (who had previously been involved with assessing one of her projects at RHUL) as a consultant to assess her ideas. In mid October 2007 a confidentiality agreement ("the NDA") was signed by Dr Hill, Mr Ohlson and Dr Porter, and Dr Porter produced a plan for the assessment of Dr Hill's ideas. That was followed by meetings in late October 2007 between Dr Porter and Dr Hill; Dr Hill's pleaded case is that at those meetings she disclosed the Close-Ended Process and the dbDNA Template Process to Dr Porter.
15. By early November 2007 Mr Ohlson had secured £75,000 investment for the proposed biotechnology company and on 8 November 2007 TGL was incorporated. Dr Hill was allocated 49% of the shares, Mr Ohlson 29% and the two other investors 11% each. Dr Hill and Mr Ohlson became directors of TGL on 16 January 2008 and Dr Porter became a director on 2 December 2008 (he had become an employee on 1 September 2008).
16. In the period between early November 2007 and January 2008 Dr Hill provided Dr Porter with various technical plans and Dr Porter provided Mr Ohlson with various assessments of Dr Hill's ideas. In January 2008 Dr Porter provided Mr Ohlson and Dr Hill with the first draft of a business plan. That went through various iterations and was eventually converted into the Information Memorandum of May 2008.
17. Dr Hill and Mr Ohlson had signed Heads of Terms in 2007, which envisaged a licence agreement between Dr Hill and TGL, but by March 2008 Mr Ohlson had decided that a service agreement, with an assignment of rights from Dr Hill to TGL, was more appropriate. A draft was sent to Dr Hill in April 2008 and after she raised an objection to one aspect, amendments were made and the Service Agreement was signed on 8 July 2008. The Service Agreement provided that it would come into effect when a certain level of investment had been secured, which occurred in early September 2008 (the precise date does not matter). As indicated above, there is a dispute as to the proper construction of the Service Agreement and the extent to which it assigned rights in the Close-Ended Process and the dbDNA Template Process.
18. In the meantime, starting in May 2008, TGL had instructed the patent attorney firm JA Kemp ("Kemps"). There were a number of meetings between TGL and Kemps and by early September 2008 Kemps had produced a draft patent application. That patent application underwent significant development (including being split into two) before filing of the priority application (and another application) on 30 January 2009. The events surrounding the interactions between Kemps and TGL and leading to the changes in the applications are disputed. Suffice it to say at this stage that the September 2008 draft did not disclose or claim the Close-Ended Process or the dbDNA Template Process, but the priority application did, and that Touchlight's case is that both inventions were conceived in early November 2008.
19. The priority application disclosed and claimed the Close-Ended Process and the dbDNA Template Process without limitation to operation under thermophilic conditions. Touchlight says that Dr Hill approved the filing of the priority application in Touchlight's name and did not suggest that she had any rights in the invention(s) disclosed and claimed by the priority application. This is the foundation for Touchlight's estoppel case. Touchlight says that, in reliance on the position taken by Dr Hill, it acted to its detriment in various ways.
20. On 9 March 2009 Dr Hill offered her resignation as an employee and CSO of TGL, raising a number of grievances. That offer of resignation was discussed at a board meeting on 20 March 2009 and accepted by the board on 3 April 2009. Dr Hill's employment formally terminated on 20 August 2009 but she remained a director of TGL until 9 November 2009.
21. On 1 February 2010 the PCT application was filed; it was published on 5 August 2010. Like the priority application, it discloses and claims the Close-Ended Process and the dbDNA Template Process without any limitation to operation under thermophilic conditions.
22. During 2009, 2010 and 2011 there were various communications between Dr Hill and TGL regarding TGL's research program and patenting activities. In early 2012 Dr Hill alleged that she was entitled to the inventions in the PCT application when not operated under thermophilic conditions.
23. TIP was incorporated on 20 October 2014 and the Third Defendant ("TDSL") was incorporated on 7 December 2015. Mr Ohlson has been a director of those companies since their incorporation, as well as of TGL. Dr Porter ceased to be a director of TGL on 30 November 2014 and ceased to be employed by TGL on 31 January 2022 (he has since acted as a consultant to TGL). EP(UK) no. 2391731 was granted to TGL on 23 January 2013 and transferred into the name of TIP on 4 October 2021. EP(UK) nos. 2612925 and 3150722 were granted to TIP on 30 November 2016 and 11 September 2019 respectively.
24. On 5 January 2021 Mishcon de Reya sent a letter on behalf of Dr Hill to the Touchlight holding company, putting it on notice that "our client considers the historic IP disputes to remain extant". On 21 November 2022 a letter before action was sent by Wiggin to Touchlight, claiming that Dr Hill was entitled to the inventions in their entirety. That claim was rejected by Bristows in a letter dated 12 December 2022. These proceedings were issued on 6 April 2023 and served on 12 April 2023.
25. The issues which I have summarised above require me to make findings of fact relating to events which largely took place in the period 2007-2009. The passage of time since those events inevitably makes it difficult to have a genuine recollection of what took place. Further, there is a danger of memories not only being lost, but being created or altered during the subsequent years. In its opening skeleton, Touchlight reminded me of what Leggatt J said about this topic in Gestmin SGPS SA v Credit Suisse (UK) Ltd [2013] EWHC 3560 (Comm) at [15]-[23] and in Blue v Ashley [2017] EWHC 1928 (Comm) at [65]-[70]. It also referred me to what Males LJ said in Simetra Global Assets Ltd v Ikon Finance Ltd [2019] EWCA Civ 1413 at [48]-[49] about the importance of contemporaneous documents in ascertaining the truth in commercial cases (citing Robert Goff LJ in The Ocean Frost [1985] 1 Lloyd's Rep. 1 at p.57 about the assistance to be gained by "reference to the objective facts and documents, to the witnesses' motives, and to the overall probabilities").
26. Mr Cuddigan accepted in his oral opening that "the Gestmin line of commentary in relation to witness memory is going to be engaged in the circumstances of this case" and "that means the contemporaneous documents have an elevated role to play". However, in its closing submissions, Touchlight reminded me of what the Court of Appeal said in Kogan v Martin [2019] EWCA Civ 1645, including at [88]:
"Gestmin is not to be taken as laying down any general principle for the assessment of evidence. It is one of a line of distinguished judicial observations that emphasise the fallibility of human memory and the need to assess witness evidence in its proper place alongside contemporaneous documentary evidence and evidence upon which undoubted or probable reliance can be placed. ... But a proper awareness of the fallibility of memory does not relieve judges of the task of making findings of fact based upon all the evidence. Heuristics or mental short cuts are no substitute for this essential judicial function. In particular, where a party's sworn evidence is to be disbelieved, the court must say why that is; it cannot simply ignore the evidence."
See also the distinction drawn at [89] between commercial cases with abundant documentation (such as Gestmin and Simetra) and cases in which it is inherently unlikely that all the interactions between the relevant persons will be fully recorded in documents.
27. In the present case, there was an order for disclosure which resulted in the disclosure of a large number of documents (at trial, the disclosure documents filled 14 lever arch files and I was told that was only a fraction of the disclosure). However, it cannot be assumed that the documentary record now available is as complete as it was at the time, and in some instances it is plainly not complete. Further, some of the key interactions took place at meetings or on telephone calls of which only summary records, or no records at all, were made. I must bear all that in mind when considering the documents which are now available.
28. When assessing the fact witnesses' evidence, I need to consider it in the light of the available documentary evidence (with the caveat about that which I have just expressed) and any facts which are agreed, and also bear in mind the risk of their evidence having been affected by the passage of time and the process of litigation, their motives both at the time and now, and the inherent probabilities.
Dr Hill's fact evidence
29. Dr Hill herself was her only fact witness. She provided two substantial witness statements and was cross-examined for about two and a half days. The length of her cross-examination was not excessive in the circumstances, nor was its tone or content inappropriate, but she plainly found the process difficult and at times distressing.
30. There were a number of unsatisfactory aspects of Dr Hill's evidence, some of which it will be necessary to address below. In the written closing submissions on Dr Hill's behalf, it was accepted that "her oral evidence is not, in the circumstances of this case, liable to assist the court save where it is consistent with the contemporaneous documents." In some cases a witness's evidence may not reliably add to the documentary record because the witness accepts that their memory of events is poor. But that was not the case with Dr Hill. She claimed to have a good memory of events. When I asked Mr Cuddigan what was meant by that statement in Dr Hill's closing submissions, he said that he was not pushing back on the inconsistencies in Dr Hill's evidence identified by Touchlight in its closing submissions, and that "I accept that her explanations ... did not reach the requisite standard for credibility before this court." Instead, he said, Dr Hill's case was advanced by reference to the contemporaneous documents. I do not believe that it is possible to airbrush out what Dr Hill said in her statements and in the witness box. Also, as Mr Speck observed, while Dr Hill's closing submissions forswore reliance on her evidence, there were instances in which continuing reliance was placed on aspects of it.
31. In Dr Hill's written closing submissions, it was said that any criticism of Dr Hill in relation to her evidence was resisted. The reasons given for such resistance related to the events of 2009 concerning the issue of inventorship, which I will address later in this judgment. It was said that it was "unsurprising that in the face of this treatment, Dr Hill was unable to give her evidence in a dispassionate and objective manner." While expert witnesses are expected to give evidence in a dispassionate and objective manner, a fact witness may understandably have a subjective and perhaps passionate view of matters, and Touchlight did not criticise Dr Hill for that. Touchlight's criticisms of Dr Hill's evidence related, instead, to its internal inconsistency, its inconsistency with the documents and its inherent lack of credibility. Further, while (as will appear below) I accept that the events of 2009 relating to the issue of inventorship added to the grievances which Dr Hill had against TGL, I do not accept that the defects in Dr Hill's evidence can be explained, let alone excused, by those events.
32. As I have said, I was not invited to regard Dr Hill's evidence as credible, and as will become clear I would have come to the same conclusion in any event in relation to many aspects of her evidence. Mr Cuddigan submitted that her memory had re-written itself over the years in a way that told her that she was right. Touchlight submitted that (with one exception) it was more likely that Dr Hill had convinced herself, over the last 16 years, of a version of events which was not correct, than that she knew her evidence was untrue. The exception related to the evidence about Dr Ali addressed below, but I am not convinced that evidence was in a different category to the remainder. Ultimately it is not necessary to form a conclusion as to whether Dr Hill knew that her evidence was untrue and I am content to adopt Touchlight's submission that it is more likely that she did not.
Touchlight's fact evidence
33. Touchlight adduced fact evidence from four fact witnesses:
(i) Mr Ohlson. While Dr Hill's closing submissions criticised Mr Ohlson for his handling of events in 2009 relating to inventorship (which I discuss below), no criticism was made of him as a witness. There were a number of aspects on which Mr Ohlson said he was unable to recall matters clearly or at all, but that is to be expected.
(ii) Dr Porter. Dr Hill submitted that Dr Porter's evidence was not satisfactory, but all the criticisms of any substance related to his claims to inventorship. I will deal with those in their proper place below, but I should say now that I do not regard that as undermining the credibility of his evidence generally. As with Mr Ohlson, on various aspects Dr Porter said he was unable to recall matters clearly or at all; again that is to be expected and I did not detect that he was saying that his memory was poor to avoid answering difficult questions.
(iii) Dr James Nicholls. Dr Nicholls joined Kemps as a trainee patent attorney in 2006 after completing an undergraduate degree in biochemistry and a PhD in biochemistry and molecular biology. He is now a partner at the same firm. At the times relevant to this dispute he was a trainee under the supervision of Mr Geoff Woods, a partner. No criticism was made of the way in which Dr Nicholls gave his evidence.
(iv) Dr Suleman Ali, a patent attorney who now works for Avidity IP Ltd but in 2008 was a partner at Kemps. Dr Ali was not called for cross-examination.
34. Part of the evidence concerned interactions between TGL and Kemps during which TGL sought and received advice from Kemps relating to patentability of various ideas and, subsequently, inventorship, and Kemps drafted patent applications on behalf of TGL. TGL claimed privilege in its communications with Kemps (see s.280 Copyright, Designs and Patents Act 1988) and Dr Hill supported that claim to privilege (she remains a significant shareholder in Touchlight). The relevant documents were produced for use in these proceedings and they were treated as confidential so that privilege was not lost, with significant parts of the trial being held in private. At the end of this judgment, I shall address the question of the extent to which redactions should be made to the public version of the parts of this judgment which deal with the materials in which privilege is claimed.
35. Mellor J gave each party permission to call a technical expert witness, with reports being served sequentially, for reasons expressed in his judgment of 8 March 2024: [2024] EWHC 533 (Pat).
36. Touchlight called Prof. Burghardt Wittig, a Professor of Biochemistry and Molecular Biology at the Freie Universität Berlin and the Chairman of a non-profit organisation called MolBio2Math. Between 1997 and 2018 he was CEO of, and then an adviser to, Mologen Holding AG. Mologen produced, and had patents relating to, so-called MIDGE vectors, which contain minimal expression cassettes flanked by two short hairpin loops added by ligation (and so resemble Touchlight's dbDNA vectors, but are produced by a different process). Dr Hill made no criticism of his evidence, and indeed relied on various aspects of it, as will appear below. Prof. Wittig was a good witness and I found his evidence on the technical aspects which I have to consider helpful.
37. Dr Hill called Dr David Mead, who is CEO of two companies that he co-founded, namely Varizymes (which focusses on developing new enzymes for amplification) and Terra Bioforge (which focusses on technologies for the capture, amplification, sequencing and over-expression of 20-150kb DNA). Between 1998 and 2016 he was Chief Scientific Officer of Lucigen, a leading developer and manufacturer of molecular biology enzymes and reagents. Touchlight submitted that Dr Mead was not a satisfactory witness in a number of respects. Dr Hill submitted that no criticism should be made of Dr Mead, but also did not rely on any of his evidence in her closing submissions. For that reason it is not necessary to examine Touchlight's criticisms of Dr Mead in detail, though I should record that there were occasions on which he seemed to be unaware of what his reports said and instances where he had to accept that his reports contained mistakes.
38. As recorded in the Order of Mellor J of 8 March 2024 the role of the experts in this case was "to address technical aspects arising in the context of [certain specified issues] and to assist with educating the court on the technology and to understand what is disclosed in technical documents". Both experts were well-qualified to fulfil that role. However both, but in particular Dr Mead, strayed beyond that role into debates about the meaning of language used in documents and expressions of opinion as to whether the documents showed, or supported the view, that Dr Hill had conceived of the processes in dispute by a particular date.
39. Another problem with some aspects of the experts' evidence was that they expressed views about what a skilled person would have understood from certain documents and sought to draw conclusions on that basis. As is well known, the skilled person of patent law differs from real people in various respects - see for example Laddie J in Pfizer's Patent [2001] FSR 16 at [62]-[63]. Of particular relevance in this case is that while the skilled person is deemed to have read a document fully and carefully and to have understood its disclosure, that may not be true of real people.
40. The parties were also given permission to call expert evidence of US law, as there was initially a dispute between them as to the law to be applied to determine entitlement in respect of the US application. Touchlight relied on evidence from The Hon. Paul Redmond Michel, a former Chief Judge of the US Court of Appeal for the Federal Circuit. Dr Hill relied on evidence from James F Haley, Jr., now of Haley Giuliano LLP but formerly a partner of Ropes & Gray and, before that, of Fish & Neave. The US law experts were not cross-examined and in the end it was common ground that it was not necessary to apply US law to determine any issue in this case; I shall therefore say no more about it.
41. Before turning to consider the evidence and the documents, it is important to be clear about what the Close-Ended Process is.
42. The first part of the Close-Ended Process involves amplification of a DNA sequence. While the process is not limited to the use of rolling circle amplification ("RCA") the parties agreed, for the purpose of these proceedings, that it could be treated as if it was. RCA starts with circular double-stranded (ds) DNA. The circular dsDNA is denatured to form circular single-stranded (ss) DNA and primers (normally random primers) are annealed to the ssDNA. A DNA polymerase then extends the primers using the ssDNA as a template. Because the polymerase has strand displacement properties, the initial result is a single-stranded linear concatemer containing repeats of the DNA sequence of the circular ssDNA. However, the polymerase will also then use the single-stranded concatemer as a template, resulting in a double-stranded linear concatemer. In fact the product will be a mixture of double-stranded concatemers of different lengths with different starting points, but that can be ignored for present purposes.
43. That was a well-known process and formed the basis for Dr Hill's original Open-Ended Process, in which the concatemers were cut into single units of dsDNA using restriction enzymes (which recognise specific short sequences in dsDNA). That required inserting restriction enzyme sites when creating the original dsDNA template that would be subject to RCA. Dr Hill illustrated the Open-Ended Process in her first witness statement like this:
44. The Close-Ended Process instead involves cutting the concatemers into single units of dsDNA using a protelomerase. Protelomerases are enzymes which recognise and act on specific long palindromic sequences of dsDNA (for example, the TelN protelomerase from the N15 phage recognises a 56 base pair (bp) palindromic sequence). Protelomerases not only cut the dsDNA in the middle of the palindromic sequence, but also close the ends by forming a phosphodiester bond between the backbones of the two DNA strands (by contrast, when a restriction enzyme is used, no such bond is formed, so the dsDNA is open-ended). In order for the protelomerase to be able to act on the concatemers, it is necessary for the original dsDNA template for RCA to contain at least one protelomerase recognition site.
45. A key aspect of the Close-Ended Process is that the protelomerase acts directly on the concatemers produced by RCA (which is why "the Direct-Acting Protelomerase Process" is a better descriptor). That is to be distinguished from a process in which the concatemers are cut into single units using a restriction enzyme (as in the Open-Ended Process) and then circularised before being treated by protelomerase, in which case the process would be what was referred to at trial as the Cut and Ligate Process.
46. When the protelomerase creates the phosphodiester bond between the two strands of dsDNA, the bases near the end of the dsDNA need to bulge out rather than hydrogen bond with their counterparts in the other strand, creating a lollipop-like structure. If this has occurred at both ends of a piece of dsDNA, the structure has a dumbbell-like or "doggybone" form.
47. If a protelomerase is applied to circular dsDNA containing a single protelomerase recognition site, the product will be a single doggybone containing the whole of the original circular dsDNA, with half of the sequence of the protelomerase recognition site at one end and the other half at the other end. If a protelomerase is instead applied to circular dsDNA containing two protelomerase recognition sites then the result will be two doggybones (each with half of the sequence of a protelomerase recognition site at each end, but with different sequences in between). If a protelomerase is applied to linear dsDNA containing one protelomerase recognition site, the result will be two lollipop-like structures or half-doggybones, whereas if it is applied to linear dsDNA containing two protelomerase recognition sites the result will be a doggybone and two half-doggybones.
48. The dbDNA Template Process is the Close-Ended Process in which the starting template for RCA is itself dbDNA. At first sight that does not appear to involve any additional insight over and above the concept of the Close-Ended Process itself because dbDNA has closed ends and so can, once denatured, act as a template for RCA. However, I agree with Touchlight that in fact it does involve an additional insight. As I have explained, dbDNA contains half of a protelomerase recognition site at each end. The insight required in order to see that it could act as a template for RCA to produce something which can be cut by protelomerase is the appreciation that, because the recognition site is palindromic, once RCA has operated on the denatured (and hence single-stranded) dbDNA, the product will contain complete protelomerase recognition sites.
49. It is important when considering the evidence and the documents to appreciate that, once the Close-Ended Process has been conceived of, it is easy to explain (it can be summarised as: put a protelomerase recognition site at a suitable point in the RCA template and use protelomerase directly on the concatemers to produce dbDNA) and to illustrate, and it is easy for someone familiar with DNA technology to understand the process from such an explanation or illustration.
50. While Dr Hill was at RHUL she used the Open-Ended Process, including as a means of amplifying DNA encoding so-called "unclonable" genes (i.e. ones that could not be amplified by plasmid replication in bacteria). One form of RCA template that she used included minimal expression cassettes, i.e. restriction enzyme sites were used to flank sections of DNA that included only the sequences needed for protein expression (promoter, gene of interest, polyA tail sequence) - the idea being that the linear dsDNA units that were cut from the concatemers and ultimately transfected into cells would lack any vector backbone sequence. She also conducted studies showing that protein expression following transfection of linear dsDNA units into cells was greater than when concatemers or open circles of DNA were transfected.
51. However, she also observed that the concatemers were prone to a problem known as "meshing" in which ssDNA being produced by RCA folds back on itself or forms more complicated structures. This can prevent the formation of dsDNA and also reduce the efficiency of cutting of the concatemers by restriction enzymes. In order to overcome the meshing problem, Dr Hill had the idea of running the RCA at a higher temperature, which would require the use of a thermophilic polymerase (one that operates at temperatures above about 45oC) rather than the polymerases normally used in RCA, such as j29 polymerase, which operate at lower (mesophilic) temperatures. She carried out initial research into the use of a thermophilic polymerase known as ThermoPhi in RCA, but that appeared to have a strong nuclease activity that degraded the concatemers.
52. None of the above background was controversial. However, virtually every aspect of Dr Hill's account of the genesis of the Close-Ended Process and the dbDNA Template Process, as set out in her pleadings and witness statements, was disputed by Touchlight.
53. Dr Hill said that in January or February 2007 she came across a paper called "Linear DNAs concatemerize in vivo and result in sustained transgene expression in mouse liver" by Chen et al., (2001) Mol. Ther. 3(3):403-410 ("Chen"). Chen reported that transfected linear DNA led to much higher expression than transfected circular DNA, and attributed that to the linear DNA forming large, unintegrated concatemers in vivo (note that this is nothing to do with the production of concatemers in vitro using RCA). Dr Hill said that she regarded Chen as raising serious concerns about the safety of using linear DNA units for gene therapy or as DNA vaccines.
54. Dr Hill said that led her to think about using closed forms of DNA which would not concatemerise in vivo, and that in March or April 2007 she did a Google search for "closing ends of DNA" and found a number of papers, including one called "Linear closed mini DNA generated by the prokaryotic cleaving-joining enzyme TelN is functional in mammalian cells" by Heinrich et al., (2002) J. Mol. Med. 80:648-654 ("Heinrich"). Figure 1 of Heinrich shows how in nature the N15 phage infects its host bacterial cell with its circular prophage DNA containing the telRL recognition site and how the protelomerase enzyme TelN cleaves the DNA and covalently closes both ends to form what Heinrich calls "doggybones".
55. In her first statement, Dr Hill said that:
"On reading Heinrich, a 'light bulb' went on, and I immediately realised that it would be possible to close the open ends of the linear DNA single units of the Open-Ended Process to make them safer for transfection. ... Moreover and beneficially, Heinrich also showed that TelN could be used to cleave the DNA at specific recognition sites, potentially eliminating or reducing the need for restriction enzymes. This was my 'eureka' moment. ... I also realised at the same time that the single units of doggybone DNA ("dbDNA") could be produced without any bacterial backbone and that as such, they would also be a perfect starting template for RCA...
Knowing that there was now a solution to overcome the problem identified in Chen, I felt I could pick up my research where I left it, specifically trying to develop an RCA process using a thermophilic strand displacement, rolling circle DNA polymerase, followed by subsequent resolution of the concatemers produced into single units of covalently closed linear DNA (dbDNA) using a protelomerase. However, I realised that to obtain the full advantages of what I already expected would result from using a thermophilic DNA polymerase to produce optimised thermophilic RCA derived concatemers (i.e., reduced meshing issues), it would be sensible to continue the resolution of those concatemers into single dbDNA units using a thermophilic protelomerase..."
56. As will be seen, in this evidence Dr Hill said that at the same time, in March or April 2007, she conceived (i) the idea of closing the ends of linear DNA units using a protelomerase (ii) the idea of creating the close-ended units by using a protelomerase (including a thermophilic protelomerase) on the concatemers produced by RCA and (iii) the idea of using dbDNA as a starting template for RCA. In other words, she had conceived the Close-Ended Process and the dbDNA Template Process. She confirmed that in her oral evidence, though she said that the "eureka moment" occurred over several days or weeks, and suggested that a further paper played a part in her thinking, namely "Mechanisms of replication and telomere resolution of the linear plasmid prophage N15" by Ravin, (2003) FEMS Microbiol. Letts. 221:1-6 ("Ravin").
57. Dr Hill said in her first statement that her eureka moment led her to reach out again to Mr Ohlson (she had first contacted him in 2006 but then put matters on hold - she said that was as a result of reading Chen) and that she brought Dr Porter into those discussions, leading to the signing of the NDA on 15 October 2007. Dr Porter then prepared a proposal for the evaluation of Dr Hill's ideas and their commercial feasibility in a number of stages, which he showed to Dr Hill and sent to Mr Ohlson on 21 October 2007.
58. It is common ground that after that date there were a number of meetings between Dr Hill and Dr Porter. In her first statement Dr Hill said:
"Given Dr Porter's role at this stage was to assess the value and viability of my ideas and to assist with obtaining patent protection over said ideas, I was aware that I needed to communicate my previous research, including my previous work with thermophiles and relating to the Open-Ended Process, and my most recent technical innovations (the Close-Ended Process and Thermophilic Close-Ended Process), in particular, as clearly and comprehensively as possible to Dr Porter in order to demonstrate to him that the innovations were worth investing in. I therefore provided Dr Porter with as much information as possible during the latter part of 2007."
59. Dr Hill said in her first statement that she particularly recalled a meeting on 22 October 2007 in which she showed Dr Porter various PowerPoints, including one she had prepared for that meeting, to illustrate how she arrived at the Close-Ended Process, and provided Dr Porter with hard copies of Heinrich and Ravin.
60. In her first statement Dr Hill had not referred to a document from Touchlight's disclosure which consists of a number of pages of notes, some in Dr Porter's handwriting and some in Dr Hill's. In her second statement Dr Hill said that Dr Porter's notes were from the 22 October 2007 meeting and that her notes were from various times but the first two pages were written for Dr Porter after the 22 October 2007 meeting (based on some notes she said she had made early that year). Those two pages included an idea for overcoming what Dr Hill regarded as the problem of concatemerisation in vivo. That idea was to ligate single-stranded DNA containing complementary sequences to the end of the expression cassettes, to allow the single-stranded region to "snap back" onto itself to produce a hairpin and so prevent the expression cassettes concatemerising.
61. Dr Hill accepted that none of the notes in that document disclosed the Close-Ended Process. She said in her second statement that she now recalled that the 22 October 2007 meeting had focussed on her old work, because Dr Porter did not want to discuss the Close-Ended Process until he had been through all her earlier work, and indeed held her back from explaining the Close-Ended Process. Nonetheless, she said she recalled pointing out to Dr Porter during this meeting "where I would replace the restriction enzymes used to produce single open-ended units from the concatemers produced by RCA with a protelomerase 56 base pair (bp) recognition site and how I would use protelomerase to cut and close the ends to produce dbDNA single units". She also said that because she was frustrated that Dr Porter was holding her back from explaining the Close-Ended Process, she "sneaked in a reference" to her new invention into her notes by "mentioning a primer and a phage to be found in the Azores (i.e. a thermophilic protelomerase)". She said that it was at a subsequent meeting that she was finally able to take Dr Porter through her new ideas by reference to a slide deck that she had pre-prepared for the meetings which showed the Close-Ended Process, and explained the dbDNA Template process to him. She added that the slide deck was one of the documents on a CD-ROM which she gave Dr Porter at one of the meetings.
62. In cross-examination Dr Hill said that she recalled these two meetings "very clearly" or at least "pretty clearly". She then said that she had gone through everything with Dr Porter at the first meeting (even though he was "recalcitrant" to her speaking about the protelomerase), explaining the Close-Ended Process and the fact that dbDNA could be used as a template, by reference to PowerPoints which were among documents on a CD-ROM given to Dr Porter. So in essence she reverted to the version of events in her first statement. When asked about the absence of any reference to the Close-Ended Process in the notes she said that she was shocked that so much was missing, including everything about the protelomerase (she said she was also shocked by having found Dr Porter going through her old notes when she came back from the toilet).
63. Dr Hill's evidence about the meetings with Dr Porter was inconsistent and implausible. She claimed in her first statement to have clear and detailed recollections of the 22 October 2007 meeting, which involved her explaining everything to Dr Porter, yet in her second statement that had been replaced with clear and detailed recollections of Dr Porter actively trying to stop her explaining her new ideas to him at that meeting, and of the new ideas being conveyed at a subsequent meeting (though still addressed at the first meeting). Given the objective of the meetings (see paragraph 58 above) it would have been inexplicable if Dr Porter had tried to prevent Dr Hill explaining her new ideas to him. Further, if at this stage Dr Hill had PowerPoint slides which showed the Close-Ended Process, one would expect them to have been in disclosure (which, as will be seen, they were not). Moreover, if Dr Hill had such slides at this time, there would have been no need to "sneak in a reference" in a note - she would just have provided the slides (even if Dr Porter had tried to prevent her speaking about the process), as indeed she said she did on a CD-ROM. And in any event, her note did not contain a reference to the Close-Ended Process or even to a protelomerase. Instead it said that the proposed thermophilic RCA reaction might be improved by the addition of "a specific phage start site encoded within the cassette template" and that "a novel thermophilic phage equivalent" might be discovered by sequencing of the Azores libraries. (I should add that there was also no reference to protelomerase in a document entitled "Vector-Free Expression Cassette Technology" which Dr Hill prepared and sent to Mr Ohlson on 24 October 2007, though it is fair to say that this document was at a relatively high level and did not address the details of the technology.)
64. None of Dr Hill's evidence about the October 2007 meetings was put to Dr Porter and, as I have said, the decision was taken not to rely on any of Dr Hill's evidence about the genesis of the inventions in the spring of 2007 and their communication to Dr Porter in the autumn of that year. I have gone through it (though not in as much detail as I would have done, by reference to other contemporaneous documents, had it been necessary to decide whether it was credible) to demonstrate that Dr Hill pleaded, and sought to support by evidence, a story of how and when the inventions were made and communicated. Dr Hill says that the decision not to rely on that story does not prevent her from succeeding in her claim, because she says it can be seen from the contemporaneous documents that the inventions were in fact made and communicated at some point before the Service Agreement came into effect in early September 2008. As Mr Speck observed, the withdrawal of Dr Hill's story in effect amounts to a withdrawal of her pleaded case. However, he did not submit that Dr Hill should not be allowed to advance a case that the inventions were made and communicated at some later point, though he did observe that the case he was now faced with was diffuse and untethered.
65. In order to address the case now advanced by Dr Hill, it is necessary to work through the contemporaneous documents in chronological order (where I quote from them, I have not corrected spelling mistakes or typographical errors). As I do so, I shall make observations on their disclosure and address evidence relating to them and points that were made about them. But I agree with Touchlight that ultimately I have to look at matters in the round and consider whether, looking at all the relevant documents and the evidence, it is more likely than not that the inventions were made before the Service Agreement came into effect.
The Stage 1 Report
66. Following his meetings with Dr Hill, Dr Porter prepared a draft Stage 1 (Technology Evaluation) Report, which he sent to Dr Hill on 6 November 2007, asking for comments and saying that he would call her the next afternoon. Dr Porter sent the Stage 1 Report, in the same form as the draft, to Mr Ohlson on 8 November 2007.
67. Project 1 addressed by the Stage 1 Report related to the production of vectorless DNA cassettes for protein expression, with potential application as DNA vaccines, in gene therapy and in cell-based protein production systems. The section on vaccines set out a number of advantages of DNA vaccines, followed by a number of problems which could be resolved by removing the need for plasmid/vector DNA and the need for bacteria to amplify the DNA vaccine cassette. The report then referred to Dr Hill's work seeking to overcome those problems, by reference to one of her unpublished papers. That work was said to show:
"1. The development of an expression cassette containing no bacterial vector sequences only the DNA required to express the required gene.
...
2. A highly efficient in vitro process for amplifying the DNA cassette that does not require the use of living cells. This is an enzymic process employing a DNA amplification technique referred to as Rolling Circle Amplification (RCA) using a commercially available enzyme, E.coli phage j29 DNA polymerase."
68. Under the heading "Intellectual Property", the report expressed the view that a thorough patent and literature search should be undertaken. It continued (original emphasis):
"Although there are aspects of Dr Hill's research results that could possibly be patented now, the real value would come from a small amount of directed research to create a stronger overall patent or patents. This would cover a new process that would overcome, in an innovative way, inefficiencies encountered at laboratory scale with the production of DNA cassettes. A strong process patent could generate significant future licensing revenue from its use in a range of applications.
Discussions with Dr Hill have identified a number of areas of research that require refinement and could strengthen and add to the value of intellectual property.
The prime objective would be to improve the performance of RCA to replicate expression cassettes (free from bacterial vector sequences) by reducing DNA mesh formation. Currently, DNA meshing reduces the efficiency of cassette formation by ~75%. Mesh formation is a result of long DNA strands binding back on itself and not with a complimentary strand, making the DNA unavailable for transcription.
Key technical objectives would be as follows:
• Reduce meshing through the introduction of single stranding binding proteins (Phi 29 DNA polymerase)
• Investigate temperature profiling to control mesh formation ( using mesophilic Phi 29 polymerase)
• Evaluate thermophilic enzyme homologues of Phi 29 over wider temperature range of reactions. Preliminary studies have aleady been undertaken. (PROKARIA ENZYME - COLLABORATION OPPORTUNITY?)
• Isolate a single stranded binding protein from Thermus sp.and evaluate with the thermophilic enzyme.
• Identify single primers for DNA amplification from a fixed point. Avoids the generation of "waste fragments" of DNA.
• Make expression cassettes (+/- bacterial vectors) for utrophin (very large and unclonable by other methods), tetanus protein C (short), GFP (Green Fluorescent Protein) and b-galactosidase. Measure efficiency of manufacture with new process.
• Transfect cassettes into cell cultures and mice (muscle tissue) and compare expression levels and longevity of expression between pairs of cassettes (+/-) bacterial vectors
• Measure levels of concatemerisation in vitro and in vivo
• Evaluate single strand tailing to prevent concatemerisation of single cassettes"
69. As will be seen, there is no mention in this report of any proposed use of protelomerase to produce dbDNA (by any process), despite the fact that the report mentions the concern about levels of concatemerisation of the transfected cassettes (which is the problem which Dr Hill said she sought to overcome by creating dbDNA). Instead, the only proposed approach to reduce concatemerisation of such cassettes is "single strand tailing". That is consistent with Dr Hill's handwritten notes, referred to above (as is the reference to identifying single primers for amplification).
70. If Dr Hill had had the idea of using protelomerase to create dbDNA vectors (by any process) by this date, it would have been surprising if she did not make any comment on the draft report when Dr Porter sent it to her, particularly if she had had any difficulty in getting Dr Porter to listen to her explanations about protelomerase at their meetings. In her oral evidence, Dr Hill said that she was shocked by the inclusion of the single-stranded tailing point instead of her inventions and called Dr Porter to complain, but he had fobbed her off and she had allowed the document to be sent to Mr Ohlson because she "just wanted the thing to move forward". I did not find her evidence on this (which was not put to Dr Porter) remotely convincing, but in any event it is no longer relied on.
The Ravin email
71. It is apparent that, as foreshadowed in the Stage 1 Report, Dr Hill and Dr Porter were conducting searches for prior art. On 20 November 2007 Dr Hill emailed Dr Porter saying: "Here is a ref to N15 prophage that produces linear molecules with telomeric like ends-a patent search here would be appreciated ......... it'd be good if we could find a thermophilic equivalent for this to include in a thermophilic RCA process." She also copied into the email the abstract of Ravin, highlighting some of the text in two sentences of the abstract, as shown by my underlining: "Upon infection of an Escherichia coli cell, the phage DNA circularises via cohensive [sic] ends. A phage-encoded enzyme, protelomerase, then cuts at another site, telRL, and forms hairpin ends (telomeres)."
72. If Dr Hill was already familiar with Ravin, and had discussed it with Dr Porter and provided him with a copy, it is hard to explain why she sent this email, and wrote it in the way she did. It is hard to avoid the conclusion that Dr Hill first saw Ravin on or just before 20 November 2007.
73. Mr Cuddigan relied on the fact that Ravin disclosed that the N15 protelomerase cut (and closed the ends of) linear DNA as well as circular DNA, and submitted that Dr Hill, as a skilled person having read Ravin, would have been aware of that fact. Much of Ms Pickard's cross-examination of Prof. Wittig was on the same premise. However, the question is not whether a skilled person who had read Ravin would have been aware that protelomerase operated on linear DNA as well as circular DNA, but whether Dr Hill had both appreciated that fact and seen how it could be employed to produce close-ended linear DNA from concatemers produced by RCA. In the absence of reliance on Dr Hill's evidence, it is necessary to consider whether any of the contemporaneous documents show such a realisation.
The Technical Plan
74. On 26 November 2007 Dr Hill sent Dr Porter a Technical Plan. Part A related to "Development of a Process for the Production of High Quality Linear DNA Expression Cassettes". It began as follows:
"The method developed up to now, involves Phi29 DNA polymerase driven rolling circle amplification, to produce concatamers from a template of an open circle form of expression cassette. There are known to be certain problems involved with the present method in that religated template DNA results in 'meshing' of the concatamers that are produced. These concatamers are not cut with restriction enzymes to produce the required linear single units. The plan described here is one to investigate potential methodologies to overcome the meshing problem and to improve the actual process for the rapid production of high quality DNA material."
75. The plan had four aspects. The first was "Replacement of Phi29 with Thermophi (Prokaria) or an equivalent thermophilic enzyme"; under this heading mention was also made of inclusion of (thermophilic) solfolobus pyrophosphatase and a thermophilic single-stranded binding protein (SSBP). The second related to determining levels of expression of linear cassette DNA in tissue culture. The third was investigating the use of a single primer system for initiation of RCA (including investigating thermophilic phages) - again consistent with Dr Hill's handwritten note referred to above.
76. The fourth aspect of Dr Hill's Technical Plan is the most important. It reads as follows:
"Addition of telomeric ends: whilst linear forms of expression cassette DNA is fine for cell factories etc, it is not adequate for DNA vaccines and gene therapy. Once inside the cell, linear DNA is known to concatamerise which will lead to greater expression due to increased gene dosage. This is unacceptable for both vaccines or gene therapy. An enzyme from phage N15 called protelomerase plus inclusion of a 56 base pair site within the expression cassette will allow the formation of linear DNA with closed ends that will not concatamerise. The revised full process for the production of linear closed DNA expression cassettes suitable for vector-free DNA vaccines and gene therapy is shown in Fig 2.
(i) Insert the 56 bp site (see Fig 1) within the expression cassette containing an egfp reporter gene and check the formation of closed linear DNA In vitro using published methodologies. Optimise this process.
(ii) Investigate the potential of obtaining a thermophilic version to produce a fully thermophilic process.
(iii) Investigate the expression levels, longevity of expression and maintenance within tissue culture.
(iv) Investigate the rate of cellular uptake of closed linear DNAs."
77. Fig. 1 of the Technical Plan is Fig. 1 from Ravin (including the original legend):
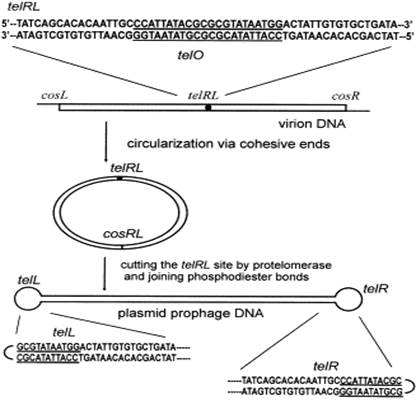
Fig. 1. Mechanism of conversion of phage DNA into linear plasmid and sequences of the telRL site and hairpin ends of the prophage. cosL, cosR, single-stranded cohesive ends; cosRL, cos site after annealing and ligation of cohesive ends; telRL, uncut target site of protelomerase; telL and telR, left and right hairpin ends of the prophage created by protelomerase. The central 22-bp ideal palindrome telO is underlined.
78. This figure illustrates how the N15 plasmid prophage DNA is created in host cells infected by the N15 phage. The linear virion DNA containing the 56 bp palindromic telRL recognition site circularises via cohesive ends and is then cut by the TelN protelomerase to produce linear DNA with closed ends (with the telL half of the recognition site at one end and the telR half at the other). Note that Ravin does not refer to that as "doggybone" DNA nor does that term appear in the Technical Plan.
79. Fig. 2 of the Technical Plan was produced by Dr Hill. It is as follows:
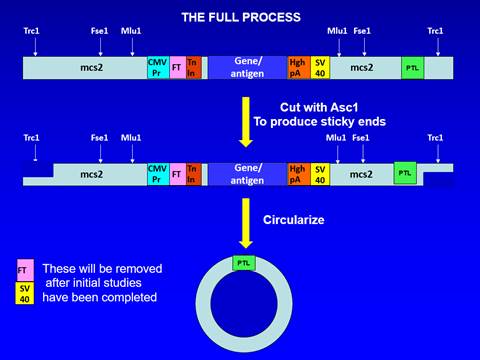
Fig 2. The revised full process for the production of linear closed DNA expression cassettes suitable for vector-free DNA vaccines and gene therapy.
80. It was common ground that Fig. 2 contained some errors. In the first slide the restriction enzyme used is said to be Asc1 but there is no Asc1 site in the DNA to be cut. More significantly, the protelomerase site (in green) is not in the right place in the DNA at the top of the first slide - if protelomerase were used on DNA having a protelomerase site between the gene of interest and the template for the polyA tail it would lead to the gene and the polyA tail being separated in the expression cassette. Further, the use of protelomerase cannot lead to a structure of the type shown at the foot of the second slide - the sequences making up each half protelomerase site should be at the two ends of the structure. These errors indicate that Dr Hill had produced the slides in a hurry and/or had not fully understood the process described in Ravin. Either explanation is inconsistent with Dr Hill having known about the action of protelomerase for some time. It is difficult to avoid the conclusion that these slides (and indeed section 4 of the Technical Plan) were prompted by her discovery of Ravin a few days earlier.
81. However, the fact that Dr Hill plainly knew about protelomerase and had proposed its use to produce linear DNA expression cassettes with closed ends that will not concatemerise in vivo does not mean that she had conceived of the Close-Ended Process. A key feature of that process is that the protelomerase is applied directly to the concatemers produced by RCA. In Fig. 2 protelomerase is shown as operating on circular DNA containing a single protelomerase recognition site. It is not shown as operating on linear DNA, still less concatemers.
82. Touchlight's case was that Fig. 2 shows the Cut and Ligate Process - the first slide showing part of a concatemer produced by RCA being cut into units using a restriction enzyme and ligated to form a circle and the second slide showing the circularised DNA being treated with protelomerase to cut it and close the ends. Touchlight pointed out that on that view there was a parallel between what happens in nature as shown in Fig. 1, and what was shown in Fig. 2, namely that in each case linear DNA is circularised and then cut with the protelomerase.
83. Dr Mead expressed the view that the circular form of DNA at the top of the second slide had not been circularised post-RCA (and so Fig. 2 did not show the Cut and Ligate Process), but he did not provide good reasons for that view and was unable to provide any convincing explanation for Fig. 2 as an alternative to that proposed by Touchlight. Dr Hill's case, as I understood it, was that the first slide in Fig. 2 shows the production of the template for RCA (the actual RCA not being depicted) and that the second slide in Fig. 2 shows what happens in nature. In closing submissions, she relied on Prof. Wittig's acceptance that it was "reasonable to think that what is going on here is you are using RCA to produce concatemers, repeating units of the expression cassette, and then you cut that repeated expression cassette with its 56 base pairs, to produce doggybone DNA". However, that was put on the premise that Dr Hill knew that a protelomerase could cut and close linear DNA, and in any event Prof. Wittig only accepted the proposition in general, before going on to say that it was not clear that Dr Hill understood the process, and to refer to later documents which he said showed the evolution of her thinking.
84. As I have said, my task is to assess all the documents and other evidence in the round. But just looking at the Technical Plan, Touchlight's explanation of Fig. 2 appears to me to be much more likely to be correct. It would be odd to include in Fig. 2 (which is supposed to show the process for producing linear closed DNA expression cassettes) a slide showing the preparation of the template for RCA (which was old) and a slide showing what happened in nature (which was already shown in Fig. 1). It is much more likely that the slides would show how the linear closed DNA expression cassettes were to be produced. If so, the slides show that taking place by the action of protelomerase on DNA that had been circularised (i.e. the Cut and Ligate Process), with the inspiration for that having come from what happens in nature as shown in Fig. 1.
85. Dr Hill's submissions drew attention to the fact that point 4(ii) of the Technical Plan said: "Investigate the potential of obtaining a thermophilic version to produce a fully thermophilic process". She suggested that this was a reference to finding a thermophilic protelomerase, and I agree that is a fair reading, especially given later documents. She then pointed out that the Cut and Ligate Process would require the use of restriction enzymes and ligases to cut the concatemers and circularise them, and if a fully thermophilic process was to be developed those would need to be thermophilic, but the Technical Plan did not envisage the need for thermophilic restriction enzymes or ligases.
86. It is correct that the Technical Plan does not mention the fact that thermophilic restriction enzymes and ligases will be needed if a thermophilic protelomerase is identified and a fully thermophilic process is to be developed. However, it was not known whether a thermophilic protelomerase could be identified. Unless and until one was, there would be no need to use thermophilic restriction enzymes and ligases. Mesophilic enzymes could be used instead (as indeed Fig. 2 envisages). Further, Prof. Wittig explained that thermophilic restriction enzymes and ligases were commercially available at the time, and their optimal reaction conditions were known. I do not find it surprising that the Technical Plan does not address the need for thermophilic restriction enzymes and ligases, if and when a thermophilic protelomerase is identified and a fully thermophilic process is to be developed.
87. Dr Hill also relied on an answer given by Prof. Wittig at the end of his cross-examination on the Technical Plan, where he agreed that "the most technical coherent reading of this document as a whole, is one that is referring to the Close-Ended Process". However, the question was put on the basis that the document has to be read "knowing what Dr Hill knows", which given the context of the cross-examination would have been understood to mean that a protelomerase could cut and close linear DNA, and on the basis that a fully thermophilic process was wanted. In any event, the assessment of the document is a matter for me having heard all the evidence, and the ultimate question does not depend on an assessment of the Technical Plan alone, but of all the documents and evidence.
The Stages 2-3 Report
88. On 29 November 2007, Dr Porter produced his Stages 2-3 Report (Intellectual Property and Market Opportunities). That report incorporated the results of prior art searches and commented on the relevance of the identified prior art to the projects and in particular to the research proposals identified in the first six bullet points in the Stage 1 Report (see paragraph 68 above). It then continued:
"As a result of extensive scientific discussion with Dr Hill, alternative designs for vaccines and gene therapy cassettes are proposed that offer improvements over the earlier described designs and processes which are subject to claims in published patent applications. A modified outline technical plan is as follows:"
89. The report then set out points 1-4 from Dr Hill's Technical Plan referred to in paragraphs 75-76 above, followed by:
"The output from this work will be a novel DNA expression cassette manufactured by an in vitro thermophilic process. Based on searches conducted to date, it should be possible to protect these ideas through patent applications. Critical to creating valuable IP will be the generation of data demonstrating the advantages of the new cassette design over existing methodologies."
That text was then followed by Figs. 1 and 2 from Dr Hill's Technical Plan.
90. The conclusion of the report began as follows:
"The main focus of this report has been the interrogation of the technical proposals from the Stage 1 Report with respect to the potential for generating commercially valuable intellectual property. The discovery of published patent applications relevant to Project 1 led to a refocus of the project objectives to get around potential issues. The aim now is to develop linear expression cassettes for vaccine or gene therapy use that are not capable of concatarmerising when introduced into a target cell. A method of achieving this has been identified and no published documents/patent applications have yet been discovered that use the methodology in therapeutic DNA cassettes. Also, and building on past experience, an in vitro thermophilic process will be developed for manufacture of the DNA cassette in a way that could generate intellectual property."
91. Neither party suggested that the Stages 2-3 Report advanced their case on the Timing Issue significantly compared to the Technical Plan.
The Heinrich emails
92. As can be seen, when the Stages 2-3 Report was prepared, it was envisaged that it might be possible to obtain patent protection both for process aspects and for the close-ended linear cassette designs themselves.
93. On 5 December 2007 Dr Hill sent Dr Porter an email with the subject "eek!". The body of the email consisted of the abstract of Heinrich. That abstract said, inter alia: "Acting on a telomere resolution site telRL, the protelomerase converts circular plasmid DNA into linear covalently closed dumbbell-shaped molecules ("doggybones") in a single-step enzyme reaction." It went on to explain that the authors had inserted two protelomerase sites into an expression plasmid to flank a gene of interest (one example being that encoding IL-12) to generate linear closed DNA which had led to expression of IL-12 in vivo.
94. On 6 December 2007 Dr Porter emailed Dr Hill to say:
"Check for patents last night on the 'doggybones' vector (I love that name!!!). They have filed a patent but not on the construct and use of the construct- only on nucleic acid expressing IL 12 and its therapeutic use.
I think we should focus on developing a thermophilic process for in vitro amplification of the doggybones cassette using proprietary reagents that we need to discover. The fall back position is that these can be sold or licensed and therefore have value."
95. As explained above, Dr Hill's invention story was that her "eureka moment" had come from reading Heinrich in March / April 2007, and that she had shown and given Dr Porter a copy of Heinrich during their meetings in late October 2007. While that story has now been abandoned, Dr Hill's closing submissions still sought to suggest that Dr Hill had given Dr Porter a hard copy of Heinrich before December 2007. However, Dr Porter's evidence was that he did not obtain a full copy of Heinrich until some point in 2008, when he asked his daughter to get hold of one for him. It should also be noted that this email exchange is the first appearance of the word "doggybones", to which Dr Porter reacted favourably. All of that points to this being the first occasion on which Dr Porter saw any part of Heinrich.
96. The most likely explanation of the Heinrich emails is that advanced by Touchlight. Dr Hill and Dr Porter were hoping that it would be possible to obtain patent protection for the linear closed DNA expression cassettes, but Heinrich showed that such cassettes had already been made and used for in vivo expression. Dr Hill found Heinrich and realised the problem that would cause, hence the "eek!". In so far as Dr Hill still contends that she was aware of Heinrich before December 2007, I reject that.
97. As with Ravin, Dr Hill's closing submissions sought to make something of the disclosure of Heinrich. In particular, reliance was placed on the conclusion of Heinrich which it was said taught that its process was superior to that used to produce MIDGE constructs, in that it used only a single step to cut and close the ends. It was said that it was unrealistic to think that Dr Hill, having read Heinrich, would have overlooked the fact that protelomerase could cut and ligate in a single step. However, as with Ravin, the question is not what a skilled person would have taken from Heinrich, but whether Dr Hill in fact conceived of using a protelomerase to cut concatemers produced by RCA to produce close-ended cassettes. Again, now that her evidence has effectively been abandoned, that has to be assessed on the basis of the documents and the other evidence.
Further Technical Plans, the Work Plan and the draft Business Plan
98. On 10 December 2007 (and again on 11 December 2007) Dr Hill emailed Dr Porter a further Technical Plan. Part 2 was headed "Development of a Thermophilic Process for the Rapid Production of 'Doggybone' Vector-Free Expression Cassettes" and contained eight numbered points. The first concerned the use of ThermoPhi in RCA, the second concerned the use of solfolobus pyrophosphatase to remove toxic end products of RCA and the third concerned the use of Thermus SSBP to reduce meshing. The fourth was as follows:
"Production of doggybone vector-free cassette to produce linear molecules with closed hairpin end using N15 protelomerase (ProTL):-
• Insert into the vector-free cassette (constructed previously) the 56 base pair protelomeric recognition site.
• Clone in a) egfp and b) lacz reporter genes into the cassette with the ProTL recognition site.
• Investigate ways to clone and express the protelomerase gene within E.coli and purify the ProTL protein for use within doggybone vector production experiments.
• Optimize production of doggybone cassettes in vitro."
Points 5 and 6 related to checking the functionality of doggybone vectors in tissue culture and in mouse thigh muscle. Point 7 was to check the ProTL enzyme activity temperature profile. Point 8 related to screening for a thermophilic phage carrying a thermophilic protelomerase.
99. This document was the one to which Dr Hill referred in her email of 7 November 2008 (see below) as having "clearly related that the Protelomerase is used to cut and ligate the ends directly after the RCA amplification step". However, the document does not do that, nor was it submitted on behalf of Dr Hill that it did.
100. Instead, reliance was again placed on the absence of references to thermophilic restriction enzymes or ligases. Dr Hill drew attention to Prof. Wittig's evidence that the plan set out "the principal work which is required to be carried out or investigated" and his acceptance in cross-examination that "if cutting and ligation reactions were intended to be part of this process, identification of suitable thermophilic enzymes would be part of the principal work in developing that process".
101. Ms Pickard's cross-examination was skilful, but in the end the assessment of the document is a matter for me taking into account the evidence (as a whole). The document clearly envisages that work will be done using N15 protelomerase, leading to the optimisation of production of doggybone cassettes in vitro and their testing in tissue culture and in mice. There is also to be screening for a thermophilic protelomerase, but it is not known whether one will be found. For similar reasons to those expressed above, I do not regard it as surprising that the document does not address the identification of suitable thermophilic restriction enzymes or ligases.
102. On 27 and 31 December 2007 and 7 January 2008 Dr Hill emailed Dr Porter various iterations of an Expanded Technical Plan. It is sufficient to consider the final iteration. Under Project 2 there were sections headed "Thermophilic pyrophosphatase (PPi) development" and "Isolate Thermophilic SSB protein". Then there was a heading "Protelomerase (ProTel)" where Dr Hill said:
"No reports of a thermophilic equivalent of this enzyme has been reported and my recent BLAST searches using both ProTel protein and DNA sequence data yielded no matches within the NCBI data base. Screening for an equivalent thermophilic phage carrying this gene could take some time especially in the light of lack of extensive hot water samples although initially screening can take place using thermophilic bacteria gene libraries in hand. Prokaria may be able to help here using their extensive stocks of thermophilic phage. Perhaps it would be sensible to come rto some arrangement for them to screen their libraries for us. In the meantime we could progress with the mesophilic version and clone this gene. The sequence is fully available and there is now a cloning vector developed by Lucigen that carries the gene within the vector. We can order a sample of this vector and PCR up the full gene and the necessary telomeric binding domain."
103. The next heading was "Modification of the Linear Expression Cassette to Allow Formation of Doggybone Vector". That included "Insertion of the TelRL (ProTel binding and cut site) into linear cassette molecule", "Addition of cos sites at ends of linear cassette" and "Check correct formation of Doggybone expression cassette with Protelomerase reaction" (where Dr Hill indicated that details of how the protelomerase was to be introduced into the cell remained to be worked out). Touchlight pointed out that the idea of addition of cos sites at the ends of the linear cassette was consistent with Dr Hill having based her ideas on Fig. 1 of Ravin, which shows that in nature the DNA circularises via cos sites before being cut with the protelomerase. I agree that the reference to cos sites does provide support for Touchlight's position. The suggestion made on behalf of Dr Hill that they could be being added merely to allow circularisation for RCA (and not be used downstream) does not ring true, given that the proposal comes under the heading "Modification of the Linear Expression Cassette to Allow Formation of Doggybone Vector".
104. At the end of the final iteration of the Expanded Technical Plan, Dr Hill added (in an update to the previous iterations): "Screening for Thermophilic phage that may carry a temperature stable protelomerase (TProTel). I am now confident after a brief literature search that there are a number of possible candidate phages that may well carry a version of this gene." This shows increased optimism compared to the original draft (see the quote in paragraph 102 above). However, it was still plainly uncertain whether a suitable thermophilic protelomerase would be identified, and the document set out plans for trying to find one, either by sampling from high temperature pools or by screening academic literature and culture collections and requesting samples, that would require several months of work. For similar reasons to those expressed above, I do not find it surprising that this document does not mention the need for thermophilic restriction enzymes and ligases if and when it proved possible to identify a suitable thermophilic protelomerase.
105. On 14 January 2008 Dr Porter created a Work Plan using Microsoft Project. As Touchlight pointed out, the steps in the Work Plan relating to protelomerase aspects are based on the final iteration of the Expanded Technical Plan, and Dr Porter said that he added timings provided by Dr Hill. Again, Dr Hill submitted that it was significant that the Work Plan did not include references to identifying or purchasing thermophilic restriction enzymes and ligases. I do not regard that as surprising for similar reasons as expressed above. Indeed it is notable that the Work Plan makes it clear that the project to screen for a thermophilic phage carrying a thermophilic protelomerase was not proposed to start until the end of September 2008 and to take until August 2009. In the meantime, it was proposed to carry out the work using mesophilic protelomerase (for which, of course, mesophilic restriction enzymes and ligases would be suitable), leading to a patent application in March 2009.
106. On 26 January 2008 Dr Porter sent Mr Ohlson and Dr Hill a draft Business Plan. In relation to Project 2: "High temperature enzymic process for producing therapeutic DNA products", it stated that:
"a number of opportunities have been identified to improve this process efficiency and generate intellectual property as follows:
◦ Develop a high temperature process for faster process reactions with benefits such as reduced risk of biological contamination
◦ Screen, isolate and test new high temperature enzymes and proteins to improve process efficiency
◦ Screen, isolate and test new high temperature enzymes to create improved vector constructs for gene delivery and expression
◦ Develop a scalable process for in vitro DNA vaccine/gene therapy production"
107. The Business Plan went through various drafts and ultimately formed the basis of the Information Memorandum which is important when it comes to the Contract Issue. Neither party said that the Business Plan advanced matters on the Timing Issue.
The Investor Presentation
108. The next document relied on by Dr Hill was a PowerPoint presentation to potential investors dated May 2008. It is not clear who created it, but it can be seen that it does have input from a technically minded person, even if generally it is pitched at a level to be accessible to non-scientists. Dr Hill's submissions relied on the fact that it envisages a high temperature in vitro process involving a thermophilic protelomerase, and pointed out that one slide includes the text "Telomeric ends introduced into linear cassette to create a "doggybone" vector" while another says "Inserts telomeric ends into linear strands of DNA". Dr Hill's submission was that the use of the words "inserts...into linear strands" was a clear reference to the protelomerase acting directly on concatemers, and reliance was placed on an answer by Prof. Wittig in support of that contention. However, Prof. Wittig's answer was on the basis of giving "inserts" what he called its "real meaning" of "inside a longer stretch". I understood him to be speaking about the ordinary use of English rather than suggesting that "inserts" had any particular technical meaning in this context. In my view Dr Hill's submission seeks to make too much of the language chosen for presentation to investors, and in any event the language is also compatible with the author meaning that what would otherwise be linear cassettes have had telomeric ends added in order to, as the slide goes on to say, prevent "unwanted concatamerisation of linear DNA vaccine constructs following injection into patient".
The 9 May 2008 meeting with Kemps and Document A
109. Following an initial meeting between TGL and Kemps on 1 April, as part of a beauty parade, on 2 May 2008 Dr Porter emailed Dr Woods some background material to TGL's research projects, including the January 2008 version of the Business Plan. It is clear (see also the Investor Presentation) that at that point TGL's view was that a fully thermophilic process would give it the best chance of patent protection.
110. The first substantive meeting between TGL and Kemps took place on 9 May 2008. There is no note of that meeting in disclosure, though Mr Ohlson said in an email on 12 May that TGL had briefed Kemps in great detail on every aspect of its technology including the future research direction in order to secure patents.
111. Following that meeting, Dr Nicholls drafted (under Mr Woods' supervision) a document intended to guide landscape searches which was sent to the search agents on 4 June 2008. It was headed Document A because there was another document performing the same task for the other TGL project (on ThermoLethal Vectors). It started by saying:
"Our client is in the early stages of planning a research project directed to developing a new process for the production of DNA vaccines. This will be a cell-free process carried out at high temperature using enzymes isolated from thermophilic organisms. The process will be used to produce DNA vaccines that are free from bacterial sequences ie are not in plasmid form."
112. The document went on to record that the main component of the process was a DNA polymerase for RCA, but that further components of the process might include a thermophilic SSBP and a thermophilic protelomerase which:
"would be used to insert telomeric ends into the linear DNA vaccine product, forming a closed "doggybone" shaped linear structure. In this structure, there are no exposed DNA ends. This is an advantageous feature for a DNA vaccine as it prevents a non-specific immune response being generated against free DNA ends in vivo. Also, it prevents integration of a linear DNA vaccine into host DNA, improving safety. Furthermore, the structure prevents concatemerisation of vaccine molecules in the host cell (which leads to over-expression of the encoded antigen)."
113. There was an argument about the language used in this passage. Dr Hill submitted (with echoes of her submission on the Investor Presentation) that "insert telomeric ends into the linear DNA vaccine product" meant that the protelomerase must be acting directly on the concatemers. Touchlight responded by saying that the "DNA vaccine product" must be the material intended for administration to patients. In my view that all involved too detailed an analysis of language used in a document which was not a draft patent application but a document intended to enable the search agents to identify relevant prior art.
114. Dr Hill also relied on the skeleton claims at the end of Document A. Claim 1 was:
"An in vitro high temperature cell-free process for production of a DNA vaccine comprising:
a) contacting a DNA template with one or more primers and a thermophilic DNA polymerase;
b) incubating the DNA template under conditions promoting DNA replication by displacement of replicated strands through strand displacement replication of another strand,
wherein the DNA template comprises a sequence of interest but is devoid of bacterial plasmid replication sequences and/or CpG motifs."
Claim 3 was:
"The process of claim 1 or 2 further comprising:
c) incubating the DNA vaccine product of b) with a thermophilic protelomerase to insert telomeric ends into the DNA."
115. Dr Hill submitted that the product of step b) would be a concatemer, with which the protelomerase is incubated in step c) to produce dbDNA. Hence, Dr Hill submitted, the skeleton claims are to the Close-Ended Process. Again Touchlight responded by noting that the starting point of step c) is a DNA vaccine product, which it said was something capable of being a DNA vaccine rather than a concatemer.
116. Again, this all seemed to involve subjecting the skeleton claims to a level of analysis which was not justified. As Dr Nicholls explained, the purpose of preparing the skeleton claims was to use words that might end up being used in claims to enable the search agents to identify keywords and classification codes, and to draft them broadly to catch as much prior art as possible.
117. Of course, Document A could only reflect the Close-Ended Process if that process had not only been conceived by May 2008 but also communicated to Kemps. Dr Hill said in her first statement that, at the first substantive meeting with Kemps, she explained that the process would be a two-step thermophilic one, involving a thermophilic polymerase for the amplification step and a thermophilic protelomerase for the step of insertion of the telomeric ends. However, Dr Nicholls said that he did not recall and did not believe that such a two-step process was discussed at the initial meeting, but instead developed as the patent applications were drafted (see further below). In his oral evidence he was clear about this. It was put to him that in her 5 June 2008 email (see below) Dr Hill was saying that the protelomerase could be used directly on the concatemers. His response was:
"That concept was never conveyed to us at any meeting with Dr Hill or in any of the project materials that I use for drafting."
118. In her second statement Dr Hill said that she believed that "we settled on the skeleton claims...through a mixture of emails and meetings" and in her oral evidence she claimed to have been "heavily involved" in the creation of the skeleton claims, including in phone calls with Kemps. However, there was no record of any such calls, Dr Nicholls did not recall any discussion with TGL about how the skeleton claims should be drafted, and it is apparent from the documents that Kemps sent Document A to the search agents without reference to TGL.
119. In her first statement Dr Hill said that at the 9 May 2008 meeting (or a subsequent meeting, though there is no further meeting that would fit into the chronology) she spoke to Dr Ali while Dr Porter was speaking to Mr Woods about something else (and Dr Nicholls was not present). She said, inter alia:
"I remember drawing out the 2-step process for him on a piece of paper and showing him how the restriction enzyme site that is used in the Open-Ended Process is replaced with a protelomerase site in the Close-Ended Process and how the action of the protelomerase on the concatemers results from the amplification step cuts the concatemers and closes the ends of the single units to form a closed "doggybone" shaped linear structure."
She adhered to this story in her oral evidence, embellishing it with descriptions of how she remembered the light coming through the window. She also said that Dr Ali had made notes of their discussions on his computer. However, as Dr Hill recognised, there was no file note of that discussion in disclosure.
120. Dr Nicholls said that it would make no sense for him not to have been at the meeting, and that he had no recollection of Dr Ali or Dr Hill conveying to him what Dr Hill said she told Dr Ali. Moreover, Dr Ali's evidence was that he had no recollection of ever doing any work relating to doggybone DNA or of the meeting which Dr Hill said he had attended. Dr Nicholls' evidence was that Dr Ali had supervised some of his work while Mr Woods was on his summer holiday, and Dr Ali's only appearance in the papers in this case was being copied in on two emails from Dr Nicholls to TGL in early September 2008.
121. Dr Hill's evidence as to what she said to Kemps is no longer said to be credible, but had it been necessary to assess the credibility of that evidence I would have rejected it.
The 5 June 2008 emails
122. On 5 June 2008, while waiting for the results of the landscape searches, Mr Woods emailed Dr Porter, Mr Ohlson and Dr Hill. The email finished:
"Finally, we have a question for Vanessa before our meeting. In the DNA vaccine process technology project, there is mention of use of protelomerase. This is used to convert linear DNA vaccine molecules into closed structures which will not concatamerise/provoke a non-specific immune response. Would the protelomerase enzyme be present during the step of DNA replication or alternatively, would it be added in a final step to convert all vaccine molecules into closed structures?"
123. Dr Hill responded 90 minutes later, saying:
"It is most likely that we will add a thermophilic version of protelomerase as a final step. We need to let the replication stage go unhindered first, then treat with the protelomerase. We may be able to include both the DNA polymerase and the ProTL together in the reaction if the required reaction temperatures allow us to do one step followed bya second step using a different temperature of incubation."
124. Dr Hill's submissions relied heavily on her reply to Mr Woods' question. It was submitted that the language used to describe the first option, "let the replication stage go unhindered first, then treat with the protelomerase", suggested that there was nothing between those two steps. Further, it was suggested that the language used to describe the second, one pot, option indicated that there were only two steps, because only two enzymes, steps and temperatures were mentioned.
125. The first point to make is that (contrary to what Dr Hill said), Dr Hill's email does not show that she had the Close-Ended Process in mind. Her submission relies once again on parsing language which cannot have been chosen with the point now under debate in mind - the question she was asked was about a different issue (would the protelomerase be present from the outset or only at the end). Prof. Wittig said that, if he had not read other documents, he could see how reading what Dr Hill said about the first option might suggest direct action of protelomerase on the concatemers. But my task is not to consider this document on its own but to assess it together with the remainder of the evidence.
126. Prof. Wittig was scathing about the one pot suggestion - regardless of whether the two-step Close-Ended Process was envisaged or the Cut and Ligate Process. His view was that including the protelomerase in the reaction mixture during RCA would hinder amplification because it would bind to the template. Of course, my task is to assess what Dr Hill meant, not whether her proposal was technically sound, but Prof. Wittig's evidence does suggest that her answer was not fully thought through.
127. In my view, the 5 June 2008 email exchange provides (at best) only slight support for Dr Hill's case, when taken in isolation. As will become apparent, in my view any such support is overwhelmed by the other materials in the case.
Further meetings with Kemps
128. The results of the landscape searches, which were broadly positive, were discussed at a further meeting on 13 June 2008. There is a note of the meeting prepared by Kemps, but neither party said that its contents advanced their case on the Timing Issue.
129. A further meeting with Kemps was held on 14 July 2008. Kemps' note of the meeting does not record who was present, and Dr Hill said that she did not recall it, but the documents show that it was rearranged specifically so that she could attend, so it is likely that she did. The note records a number of points under the heading "DNA vaccine process technology: Process patent" including:
"a) Number of essential method steps; order in which they can be performed.
b) Reaction components - enzymes/other proteins; all publicly available? If not, how can be obtained e.g thermophilic protelomerase. Reaction conditions - basic protocol/requirements for each element to work
c) Structure of template; basic elements of expression construct.
d) Structure of initial RCA product; downstream processing to give final product."
It therefore appears that all these matters were discussed at this meeting. If Dr Hill had the Close-Ended Process in mind, it is inconceivable that it would not have been discussed.
130. Following that meeting, Mr Woods indicated that Dr Nicholls would start work on drafting the patent applications and would send them out for review by Dr Hill and Dr Porter at the start of August (in fact, as will appear, the drafts were not circulated until early September, after Dr Nicholls returned from holiday).
The August 2008 slide deck
131. Dr Hill's disclosure included a PowerPoint slide deck which she last updated on 29 August 2008. The title slide is "Rapid Production of Novel Improved DNA Vaccines and Gene Therapy Products".
132. The slide deck starts with some slides about vaccines in general, and DNA vaccines in particular, and identifies some problems with DNA vaccines and how they may be overcome by using vector-free expression cassettes produced in vitro. After a slide showing the expression cassette design (which envisages but does not show a protelomerase recognition site) the deck turns to consider the production process, explaining why a thermophilic process should be used.
133. Then these two slides appear:
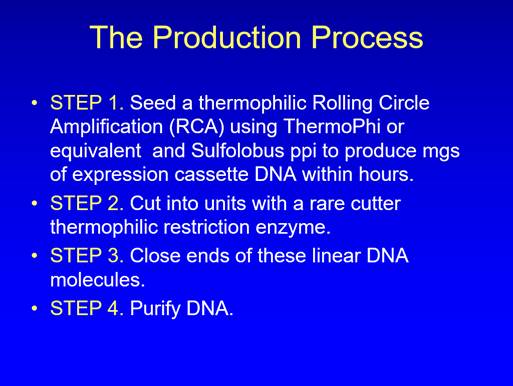
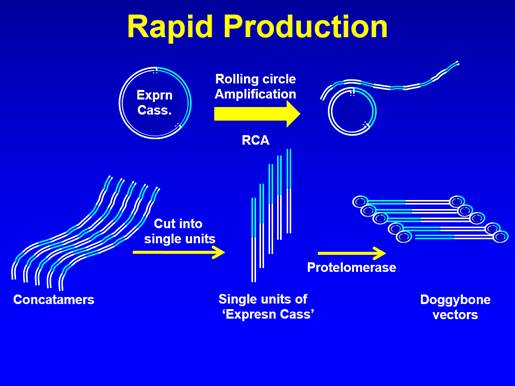
134. The process shown and described on these slides involves a step (step 2) of cutting the concatemers into single expression cassette units with a thermophilic restriction enzyme and a step (step 3) of converting those single units into dbDNA with a protelomerase. That process is not clearly the Cut and Ligate Process, in that circularisation of the single units is not depicted or described, but it is not inconsistent with that process. Also, as Prof. Wittig pointed out, in order for a protelomerase to convert linear single units into doggybones, without an intermediate step of circularisation, the linear single units would need to carry two protelomerase sites, but only one is shown in the slide showing the construction of the expression cassette.
135. However, these slides are clearly inconsistent with the Close-Ended Process. Dr Hill's attempts to explain them were not remotely credible. She suggested that the first slide was an old one (from when she was working on the Open-Ended Process) which she had not finished editing and so had failed to remove step 2 when adding step 3. That in itself was not credible, but it is also inconsistent with what she had added for step 3: "Close ends of these linear DNA molecules". If she had intended there to be a single step between RCA and purification, it would not have involved closing the ends of "these" linear DNA molecules but cutting the concatemers and closing the ends.
136. In his first report, Dr Mead suggested that the second slide:
"either shows the concatemers being cut by a restriction enzyme and then the open ends closed using protelomerase (which would require two protelomerase target sites in the template) or it shows that the protelomerase first cuts the concatemers before going on to close the open ends of the single units to create the doggybone vectors (which would require one protelomerase target site)."
137. However, in cross-examination he accepted that he had made a mistake in proposing the second explanation - protelomerase cuts and closes at the same time, so there would be no intermediate single units if protelomerase was being used to cut the concatemers.
138. Dr Hill's closing submissions sought to suggest that these slides might represent an alternative line of thinking, developed after or in parallel with the Close-Ended Process. That submission suffered from a number of problems, including that there was no evidence to support it and that it would be surprising if the alternative appeared in the slide deck but the Close-Ended Process did not, particularly as Dr Hill's case was that the Close-Ended Process was plainly superior to that shown in the slide deck.
139. This is a convenient point at which to address the submission made by Dr Hill that the Cut and Ligate Process "makes no sense as a technical matter". The point was really that the Cut and Ligate Process requires additional unnecessary steps. That is correct if one has conceived of the Close-Ended Process, but they would not be perceived as additional or unnecessary if one has not done so. Further, the point was taken that Dr Hill must have had the Close-Ended Process in mind because she was trying to develop a rapid production process. However, the second slide in paragraph 133 above is headed "Rapid Production" but shows a process which is not the Close-Ended Process.
140. The next slide in the deck explains that a requirement for "Step 4" is "a method of closing the ends of our DNA expression". Dr Hill sought to suggest that this slide did not follow on from the previous ones, and related to requirements for her two step process. However, the natural reading of this slide is that it relates to requirements for the process described on the previous two slides, with "Step 4" being a typo for "Step 3" and the word "cassette" missing after "expression". That is consistent with the next slide which is headed "Closing the Ends of the Expression Cassette" and explains that "Protelomerases do just that". That slide is followed by slides explaining what ProTL is and that it can be used to produce "doggybone" expression cassettes, and that TGL proposes to identify thermophilic protelomerases.
141. The deck then contains a slide showing Fig. 1 of Ravin followed by two slides which are similar to those in Fig. 2 of the Technical Plan (with the locations of the protelomerase sites corrected):

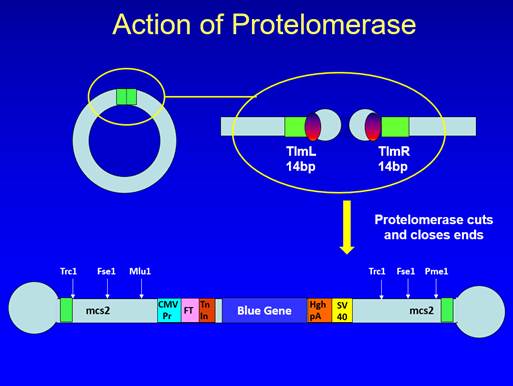
142. I have commented on the equivalents of those slides when considering the Technical Plan. Those slides are then followed by these two:
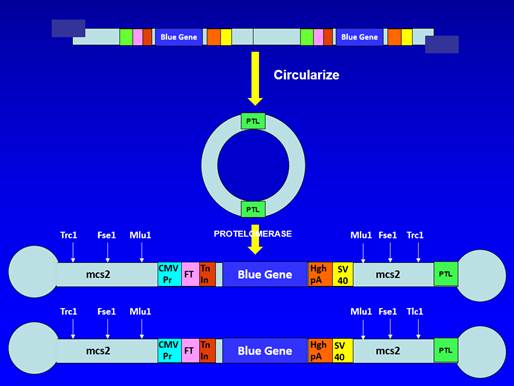
143. The first slide of this pair is an annotated version of Fig. 3 from Ravin, explaining ways in which the dbDNA is processed in nature. The second slide appears to draw inspiration from the Ravin figure and show how circularisation of a pair of expression cassettes followed by the action of protelomerase can lead to the production of two doggybone molecules. Dr Hill's explanation of this slide was not remotely credible. She began by suggesting that it showed her "mulling over this concept of using dbDNA as a template" but the slide does not show the use of a dbDNA template. Then she suggested that the top of the slide showed the creation of a template for RCA which produced two copies of the expression cassette every time the polymerase went round the circle. But apart from the fact that there is nothing to suggest that the top part of the slide shows the creation of an RCA template (it would not make sense to apply protelomerase to that template) she was unable to provide a coherent explanation of why it would make any difference to the production of concatemers whether the RCA template contained one or two copies of the expression cassette.
144. This set of slides provides powerful evidence in support of Touchlight's case. It is consistent with Dr Hill having the Cut and Ligate Process in mind and inconsistent with her having the Close-Ended Process in mind. However, as I have said about other documents, these slides are just part of the picture, and the documents and evidence must be considered as a whole.
The September 2008 draft patent application
145. On 5 September 2008 Dr Nicholls sent Dr Porter, Mr Ohlson and Dr Hill a draft of the DNA vaccine process patent application which he described as "focussing on the various concepts that are encompassed in [TGL's] process technology". He explained that he had included the doggybone (closed linear DNA) concept and had added claims to doggybone expression constructs as DNA molecules per se, and to their use in therapy. He then set out various questions regarding technical aspects of the process, including whether thermophilic restriction enzymes would be of particular use after the RCA step. He concluded by saying that he looked forward to comments and answers on his questions, together with input in describing the invention in a greater level of detail.
146. Of relevance to the Timing Issue is that, on pages 5-6 of the draft application, the following passage appeared:
"As outlined above, strand displacement reactions, in particular those using rolling circle amplification, produce a continuous series of tandem units of the amplified DNA (a concatamer). In many embodiments, the amplified DNA will be required for use as a single unit, and so such concatamers require processing to release single units of the amplified DNA. Accordingly, where the amplified DNA comprises concatamers comprising tandem units of DNA sequences amplified from the DNA template, the process may further comprises the additional step of c) cutting the concatamers to produce single units of amplified DNA sequence.
Resolution of concatamers will typically be carried out by cutting with one or more restriction endonucleases. Typically, a restriction endonuclease will be selected to cut at a single site in the amplified DNA. Where the DNA template houses a gene or other DNA sequence of interest, the DNA template may be selected to contain one or more rare-cutting restriction endonuclease sites in the region outside the gene or other DNA sequence of interest. Rare-cutting restriction endonucleases are enzymes whose recognition sites are found in low frequency in genomic DNA. Examples include [Examples of rare cutters]. Use of rare cutting restriction endonucleases is selected to avoid excision in the resolution step of a portion of the amplified DNA e.g. an expression cassette or gene which is required to be retained in the final product.
In some embodiments, the cutting step will also be carried out at a thermophilic temperature for optimum efficiency. In such embodiments, restriction endonucleases which are functional at thermophilic temperatures will be used. Examples of such restriction enzymes include [Insert thermophilic restriction enzymes]. Alternatively, the product mixture may be cooled to a lower temperature to allow use of other types of restriction endonuclease.
Following resolution into linearised single units, there may be a step of religation of the linearised DNA so as to form a closed circular DNA (ccDNA). Preferably, a DNA ligase enzyme may be used in this step. The ccDNA may then be subjected to supercoiling by incubation with a topoisomerase enzyme."
147. Then, on pages 8-9, the following passages appeared:
"Accordingly, the invention provides for excision of the expression cassette from DNA amplified by the above process. Typically, this requires the presence of restriction endonuclease sites flanking the expression cassette on either side. As above, such sites may be recognised by rare-cutter restriction endonucleases to minimise the possibility of cutting within the expression cassette.
...
In a related embodiment directed to production of DNA molecules of improved safety, there is a step of incubation of single units obtained according to the above process with a protelomerase (ProT) enzyme, optionally bacteriophage N15 ProT. In one embodiment, the linearised single units obtained following resolution of the concatamers produced in the amplification step of the process are religated to form ccDNA but are then converted into closed linear DNA molecules by the action of ProT. In other embodiments a minimal expression cassette excised from the single unit may be incubated with ProT. ProT is able to introduce telomeric ends into DNA. Molecules generated by ProT have a dumb-bell structure shaped with left and right hairpin ends flanking a central double-stranded core.
Thus, such molecules are not circular, but are "closed linear "in structure. The term "closed linear" describes a structure where the base-paired double stranded region is covalently linked at either end by the hairpin loop. This may be contrasted with an "open linear" structure where the two strands in the double stranded DNA are only bonded together by base pairing interactions.
Formation of the closed linear structure typically requires the presence of a telRL target site for ProT in the single unit. In particular, the closed linear molecule may have hairpin ends comprising telL and telR sites flanking a central double-stranded region. The hairpin ends may comprise regions of DNA where conventional Watson-Crick base pairing is not present. [More detail needed here about mechanism of action of ProT; definition of telL sites, other types of ProT].
The incubation step with ProT, and the production of closed linear DNA molecules, is selected to prevent the presence of exposed DNA ends where a linear DNA molecule is required for the therapeutic application of interest. [Why are linear molecules preferred over circular molecules?] Absence of exposed DNA ends increases the stability of the DNA molecule and prevents generation of a non-specific immune response against the exposed termini. Also, the chances of integration of the linear DNA into the host chromosome are greatly reduced. Furthermore, the structure prevents concatamerisation of the DNA molecules in the host cell. Concatamerisation in cells leads to increased gene dosage and consequently greater expression, so its prevention allows for better control of expression level."
148. Claim 1 was, in summary, to an in vitro amplification process conducted at 50-90oC employing a thermophilic polymerase and a thermophilic SSBP. Claim 3 was to such a process in which the product comprises concatemers and the process comprises the additional step of cutting the concatemers to produce single units. Claim 4 was to such a process where the cutting is carried out by restriction endonucleases. Claim 5 was to a process of claim 3 where the single units are ligated to form circular DNA molecules. Claim 9 was to a process according to any of claims 3 to 8 further comprising incubation of the single units with a protelomerase, optionally from N15. Claim 14 was a product claim, to closed linear DNA having hairpin ends comprising telL and telR sites flanking a central double-stranded region having certain characteristics (and lacking others).
149. As will be seen, the draft patent application clearly disclosed and claimed the Cut and Ligate Process and neither disclosed nor claimed the Close-Ended Process. That is consistent with Dr Nicholls' evidence that Kemps had not been told about the Close-Ended Process by this stage.
150. Dr Hill's case is that Kemps had been told about the Close-Ended Process in May 2008 - that is the basis for her contention that the Close-Ended Process can be seen in the skeleton claims in Document A. So her case has to be that Dr Nicholls had forgotten about the Close-Ended Process by the time he came to write the draft patent application, and had replaced it with the Cut and Ligate Process (she submitted that it was Dr Nicholls who had come up with that idea). That is inherently unlikely. Dr Hill pointed out that Dr Nicholls was still training as a patent attorney and that, at the time the draft patent application was circulated, Mr Woods was on holiday (it was at that point that Dr Ali was copied in on emails). However, Dr Nicholls' evidence was that he drafted the application with guidance from Mr Woods and that he started with the claims, on which Mr Woods was "fairly pernickety". So for the patent application to have ended up in the form it did would require not only Dr Nicholls, but also Mr Woods, to have forgotten about the Close-Ended Process, which is even less likely.
151. In support of the suggestion that Dr Nicholls had somehow forgotten the Close-Ended Process and come up with the Cut and Ligate Process, Dr Hill pointed out that the draft patent application does not contain the one pot possibility which was mentioned in her email of 5 June 2008 (a possibility which does appear in the PCT application). However, Dr Nicholls said that his recollection was that at the time they had understood from the email that the protelomerase was most likely to be used as a final step. I cannot see how the omission of the one pot option from the September 2008 draft is an indication that Dr Nicholls had forgotten the Close-Ended Process and come up with the Cut and Ligate Process.
Dr Porter's October 2008 note
152. Dr Hill placed reliance on a note written by Dr Porter on 15 October 2008. Dr Porter could remember very little about this note or what he was thinking when he wrote it, and I do not find that surprising. Dr Hill relied on the fact that the bullet points under the heading "Process Components" included "Thermophilic endonucleases. Cutting sites? Efficiencies and optimal conditions for activity" and "Thermophilic ligases for producing circular double stranded DNA". Dr Hill drew attention to the fact that no consideration had been given to thermophilic restriction enzymes or ligases in the various Technical Plans and the Work Plan in December 2007 - January 2008 and submitted that Dr Porter had only started to think about them in October 2008 because he had seen the draft patent application which, for the first time, disclosed the Cut and Ligate Process, as well as Dr Nicholls' questions.
153. I agree that it is likely that Dr Porter was prompted to address thermophilic restriction enzymes and ligases by the draft patent application, including the parts in square brackets which required input, and Dr Nicholls' questions. But that does not mean that he was not already aware of the Cut and Ligate Process, nor that Dr Hill did not have that process in mind at the time. In that regard it is notable that in her August 2008 slide deck, the slide on the production process has as Step 2: "Cut into units with a rare cutter thermophilic restriction enzyme" (see paragraph 133 above).
The 27 October 2008 meeting
154. On 16 October 2008 there was a TGL meeting attended by Mr Ohlson, Dr Hill and Dr Porter following which an action list was prepared. The actions included Dr Hill to review the draft patent application by 22 October, Dr Hill and Dr Porter to prepare additional information for completion of the draft by 24 October, Dr Porter to arrange a meeting with Kemps relating to the patent application by 24 October and Dr Hill, Dr Porter and Kemps to complete the draft patent application by 31 October. Another TGL meeting was scheduled for 24 October.
155. On 17 October Dr Porter emailed Dr Hill saying: "Could you please carefully review the attached patent application and Jimmy's [Dr Nicholls'] comments and questions so that we can discuss week ahead of Friday's meeting." Dr Hill replied "Will do".
156. On 20 October Dr Nicholls chased Dr Porter for comments and answers to his questions and a meeting was arranged at Kemps on 27 October. On 24 October Dr Porter emailed Dr Hill to check that she could make the meeting, saying "I have done as much preparation as I can this week but will need your technical input to ensure the accuracy and completeness of the application and that the strategy is correct. I have quite a few things to sound you out about, so if we could meet an hour earlier over coffee, that would be great." Dr Hill replied to say that she would be at the meeting, and arrangements were made for her to meet Dr Porter an hour in advance.
157. Kemps' hand-written notes of the meeting of 27 October were disclosed. They include: "Substrate for ProT needs to be covalently closed i.e. circular, Vanessa to confirm this". Similarly, Kemps' letter of 3 November 2008 summarising the matters discussed at the 27 October meeting says: "Please can you also confirm whether it is appropriate for claim 9 to be dependent on claim 3, or if in practice ProT only works on religated circular templates (claim 5)."
158. It therefore appears that at the meeting of 27 October a question arose as to whether protelomerase required a circular substrate, and that the outcome of the discussion was that it did, but Dr Hill would confirm. I agree with Touchlight that if Dr Hill had been aware that protelomerase also operated on linear DNA and so did not need a circular substrate, it is surprising that she did not mention that at the meeting when the question was posed.
159. Dr Hill's explanation was that she arrived at the meeting unprepared, having not read the draft patent application in advance, and that she was baffled and confused by some of the questions raised at the meeting.
160. As can be seen above, Dr Hill had agreed at the meeting on 16 October 2008 to review the draft patent application by 22 October and had confirmed that she would review it in response to Dr Porter's email on 17 October 2008. However, she said that she had not done so before the meeting with Kemps on 27 October because she had been very busy. It is clear that Dr Porter had been having difficulty getting Dr Hill to engage with the draft patent application and Dr Nicholls' questions because she had claimed to be busy. However, I did not understand Dr Porter's evidence to establish that Dr Hill had not considered the draft patent application at all before the meeting with Kemps. It would be very surprising if Dr Hill, who was well aware of the importance of patent protection for TGL, had not considered the draft patent application before a meeting with Kemps to discuss it, particularly as she had agreed to do so and to meet Dr Porter in advance to provide technical input to ensure its accuracy and completeness.
161. However, even if Dr Hill had not read the draft patent application in advance of the meeting, it is hard to see why she would have been baffled and confused by the question of whether protelomerase needed a circular DNA substrate. That would have been an easy technical question for Dr Hill to answer (if she knew the answer) regardless of whether she had read the draft patent application and regardless of whether she knew the reason for the question being asked.
162. In closing submissions on behalf of Dr Hill, a number of alternative explanations were suggested for why she might not have provided the correct answer to the question at the meeting. It was suggested that the gender "authority gap" should be considered, i.e. that a woman may be less confident than a man in answering such a question without checking the answer. I recognise that in some cases that may well be so, but I saw nothing in Dr Hill's performance in the witness box nor in the contemporaneous documents to suggest that as a plausible explanation in her case. It was also suggested that Dr Hill might have been tired and not concentrating or might have misheard or misunderstood. However, Dr Nicholls' notes show Dr Hill participating at various points in the meeting, so she was plainly engaged in the discussion, and having seen her in the witness box it seems highly likely that if Dr Hill had been in any doubt about what she was being asked she would have asked for clarification.
The 5 November 2008 call and the 7 November 2008 email
163. On 4 November 2008 Dr Porter emailed Dr Nicholls to thank him for the meeting summary, saying that they would start to put together the additional information that he had requested. He also emailed Dr Hill to confirm that they were due to meet on 10 November to prepare a response to Kemps and to ask Dr Hill if she could "come up with some "doggybone" designs based on some of your thoughts at the Kemp meeting".
164. On 5 November 2008 Dr Porter called Dr Nicholls. Dr Nicholls' file note of that conversation starts as follows:
"Neil telephoned to discuss the "improved expression cassette" concept. He indicated that following his review of the mechanism of action of protelomerase (ProT), Touchlight would want to cover an additional aspect in the proposed application. ProT has a combined endonuclease and ligase activity, such that it can cleave and rejoin double stranded DNA molecules without the need for separate use of restriction enzymes and DNA ligases. Thus, we should add a claim to a process for making the expression cassette molecule which involves the direct generation of the expression cassettes from material amplified by DNA polymerase. In particular, where the DNA application is rolling circle, the concatamers that are generated can be directly resolved into the expression cassette molecules using protelomerase and no other resolving enzymes."
165. As will be apparent, what Dr Nicholls records Dr Porter as having described on this call was the Close-Ended Process. Dr Nicholls' evidence was that this was the first time that the concept of having the protelomerase act directly on the amplified material was raised, and that he recalled thinking that [xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx xxxx] His recollection of his reaction about [xxxx xxxx xxxx xxxx xxxx xxxx] is inherently plausible and is consistent with his recommendation, also recorded in his file note, to separate off protelomerase aspects to a separate application.
166. Dr Porter had no real recollection of the events surrounding this call. Dr Hill's evidence fluctuated somewhat, but the thrust of it was that shortly before 5 November she and Dr Porter had sat down at the end of the lab to run through the draft patent application (she said that was the first time she had read it) and she was shocked to notice that the document contained material based on her draft RHUL papers and mistakenly referred to ligation of the single units before the protelomerase step. She said she told Dr Porter that needed to be corrected, and that they had a further conversation by telephone about it on 5 November, at which she reiterated the mistake and the need for correction. She said that Dr Porter was embarrassed, tense and angry but promised to call Kemps to explain the position.
167. On Friday 7 November 2008 Dr Hill emailed Dr Porter as follows:
"After our conversation Wednesday, I realised that we had gone a bit off track with the RCA patent. I suspect this is due to such a long break since we last saw Jamie [sic] and being so thinly spread with so much other stuff to do at this time. I think what he has done, is concentrated on the RCA papers I wrote and has not looked at the technical plan we gave him. The papers really relate mostly, to how to clone something into our linear cassette and the technical plan relates to the actual vaccine production process. The original technical plan (attached) from Dec '07 clearly relates that the Protelomerase is used to cut and ligate the ends directly after the RCA amplification step and hence we did not propose to use restriction enzymes or a ligase. In the light of this and the new idea of splitting this patent into 2, I think we need to sit down together with the 3 separate areas of work (the 3rd being the cassette design) and work out how best to organise these 1/2 patents. Once we have sorted this one then we can move to the TLVector system. Hope this all makes sense!"
168. On its face, this supports Dr Hill's case. She is saying that the December 2007 Technical Plan involved using protelomerase to cut and ligate the ends directly after the RCA step, without the need for restriction enzymes or ligases (though as I have said above, that Technical Plan does not in fact "clearly relate" that) and she presents an explanation of how the draft patent application ended up saying something different. It should be noted that this email puts the date of conception of the Close-Ended Process as being before the (second) Technical Plan which was first emailed by Dr Hill to Dr Porter on 10 December 2007.
169. The 7 November 2008 email is different from the other documents of significance in this case. The other documents are valuable for their record of what people said to others at the time, or because they record what people were proposing as technical matters at the time. In the case of this document, the question is whether its contents were true at the time Dr Hill wrote it, or whether there is another explanation for its contents. One possible explanation was suggested by Touchlight: once it had been appreciated that protelomerase could be used directly on the concatemers, Dr Hill could not believe that she had not spotted that earlier, her pride was hurt, and she sought to create an alternative version of events. That explanation was squarely put to Dr Hill, though she rejected it.
170. In this regard it is important that Dr Hill no longer relies on her own evidence in these proceedings. The evidential value of the 7 November 2008 email in supporting Dr Hill's case is much reduced in circumstances where there is no reliable evidence from Dr Hill that its contents were true when she wrote it, and there is a credible alternative explanation.
171. Dr Hill also relied on the fact that, in Dr Porter's response to the 7 November email in an email later that day, he did not take issue with what Dr Hill had said, but instead said: "We can discuss these patent options when we meet on Monday. I agree that it is complicated but things are coming together. I also agree with you that we should discuss and agree the patenting strategy ie number of patents etc." However, Dr Porter said that at the time he was concerned about getting the patent application drafted and filed.
172. Dr Hill submitted that it was critical to Touchlight's case that it was Dr Porter that conceived of the Close-Ended Process in early November 2008. It was said that there were only two options - Dr Porter conceived the invention then and called Kemps (either before or after speaking to Dr Hill about it) or Dr Porter discussed matters with Dr Hill and called Kemps having appreciated that the draft patent application had failed to reflect the Close-Ended Process which had been conceived of by Dr Hill long before then. I do not agree that those are the only two options or that Touchlight's case relies on the first option being true. Touchlight's case is that the Close-Ended Process was conceived of by Dr Hill in early November 2008. That is not inconsistent with Dr Porter then calling Kemps to tell them about it following a discussion with Dr Hill.
The 12 November 2008 call
173. On 12 November 2008 Dr Porter called Dr Nicholls. Dr Nicholls' manuscript notes of the conversation were disclosed, as was his file note. The file note records that Dr Porter had called to update him following a TGL meeting on 10 November and goes on to say:
"In regards to the second patent application (improved expression cassette; our ref: N.106698), he indicated that Touchlight were particularly excited about this project. The process Touchlight have in mind is using a closed linear DNA molecule as a starting template, which would then be denatured and amplified, preferably by RCA. The denaturation step converts the closed linear molecule into a circular molecule, and so RCA can be used to amplify these circle forming long concatamers.
The key point is that ProT can be used to resolve such concatamers directly without need for additional enzymes, as discussed in our 5 November 2008 phone call. [xxxxxxxxx xxxxxxxxxx xxxxxxxxxxx xxxxxxxxx xxxxxxxxx xxxxxxxxxxxxx xxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxx xxxxxxxxxxxxx xxxxxxxxxxxxx xxxxxxxxxxx xxxxxxxxxxxx xxxxxxxxxxx xxxxxxxxxxxx xxxxxxxxxx xxxxxxxxxx xxxxxxxxxx xxxxxxxxx xxxxxxxxxx xxxxxxxxxx xxxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxxx xxxxxxxxx xxxxxxxx xxxxxxx xxxxxxxx xxxxxxxxx xxxxxxxx xxxxxxxxx xxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxxx xxxxxxxxx xxxxxxxxxxxxxxx]"
174. The manuscript notes are consistent with the file note. They include "Linear DNA with telomeric ends as starting material ... Heat -> converts doggybone -> circular molecule in cassette, have Pro T binding sites ... [then do RCA via mech of 1st patent - long concatamers of original material] ... Pro T resolves directly (no need for extra enzymes)".
175. This is the first record of the idea of using doggybone DNA as a template for RCA and accordingly of the dbDNA Template Process. Dr Nicholls' evidence was that this was, as far as he could recall, the first he had heard of this concept, and that is consistent with his contemporaneous notes.
The November 2008 slide deck
176. On 14 November 2008 Dr Hill sent Dr Porter and Mr Ohlson a new set of PowerPoint slides. After a series of slides relating to expression cassette designs and how they could be created, this slide appears:

177. This slide, like the notes of the conversation between Dr Porter and Dr Nicholls on 12 November, contains the idea of using doggybone DNA as starting material for RCA.
178. There are then a series of slides relating to the production process which are plainly derived from slides in the August 2008 slide deck. Importantly, they include this slide:
179. This slide should be compared with the corresponding slide in the August 2008 slide deck, which is the top slide in paragraph 133 above. Steps 2 and 3 in the August 2008 slide deck have been replaced with a single step: "Cut and close ends of these linear DNA molecules with a thermophilic protelomerase". Similarly one of the other slides from the August 2008 slide deck (not reproduced above) refers to the production process being "an entirely thermophilic process"; in the November 2008 slide deck that has become "an entirely thermophilic, 2 step process".
180. Plainly this slide deck is supportive of Touchlight's case. The differences between the comparable slides in the August 2008 and November 2008 slide deck strongly suggest a change in the proposed process, from one in which the concatamers were cut with restriction enzymes before the ends of the single units were closed using protelomerase to one in which protelomerase was used directly on the concatamers to cut and close the ends. Further, it is consistent (as are Dr Nicholls' file notes and evidence) with the Close-Ended Process and the dbDNA Template Process having been conceived in early November 2008. Dr Hill's attempts to explain the changes between the August 2008 and November 2008 slide decks were not at all credible.
The 2009 slide deck
181. The disclosure contains copies of a slide deck prepared by Dr Hill with the file name "Research Update Jan 09". The copies are dated April 2009 and December 2009, but the precise date in 2009 on which the slide deck was created does not matter for the purpose of the Timing Issue. The first slide in the deck is as follows:
182. This slide should be compared with the corresponding slide from the August 2008 slide deck, which is the second slide shown in paragraph 133 above. As can be seen, in the 2009 slide deck the process is shown as involving a single step in which the protelomerase produces doggybone vectors from the concatemers. That is consistent with the way in which the process is described in the slide from the November 2008 slide deck in paragraph 178 above. By contrast, in the August 2008 slide deck that process requires two steps. Again, the difference between the August 2008 slide deck and the 2009 slide deck supports Touchlight's case.
183. Dr Mead made the point that the 2009 slide deck also contained, towards the end, slides from the August 2008 slide deck, and suggested that showed that there was no change in thinking between the two. That was a bad point. The 2009 slide deck does indeed contain, at the back, slides from the August 2008 slide deck and also the November 2008 slide deck. The obvious explanation for that is that, as Dr Hill explained, she would sometimes add to her slide decks to create later versions (and she specifically said that was how the 2009 slide deck came to be created). However, the slide from the August 2008 slide deck which corresponds to the first slide in the 2009 slide deck is not included at the back of the 2009 slide deck. Plainly, it has been amended to create the first slide of the 2009 slide deck.
184. The final episode which it is necessary to consider in relation to the Timing Issue is the dispute about inventorship which arose after the priority application had been filed on 30 January 2009. This was relied on heavily by Dr Hill in her closing submissions.
185. The issue appears to have been prompted by Mr Woods sending Mr Ohlson a letter on 19 February 2009 noting that the priority application had been filed without a statement of inventorship and asking him to consider whether anyone else, apart from Dr Hill, qualified as an inventor (providing Kemps' circular on inventorship by way of guidance). He also recommended that the inventor(s) should execute a confirmatory assignment in favour of TGL and provided a suggested draft.
186. It appears that by 5 March 2009 Dr Porter had been claiming that he should be named as an inventor. Early that day Mr Ohlson emailed Dr Hill saying:
"I had a chat with Neil regarding inventorship as we previously discussed yesterday. I think the first thing to say is that Neil is a grown up lad, is aware of the sensitivities around the subject, and completely recognises that Touchlight is a result of your lifetimes work. In me asking him the question 'what bits of the patent do you think you invented', he responded with 'do you (vanessa) think that we would have got the initial ideas into a patentable form without Neils contribution'. If the answer to that is 'yes', then he will totally accept it. I think he thinks that it would be 'nice' to acknowledge his contribution.
...
So please just have a little think about this. Clearly it is your work, and I will go with whatever you think. And am sure Neil will too. I just want to get the whole patent area tidied up."
187. Later that day Dr Hill emailed Mr Ohlson attaching the RHUL inventorship guidelines and saying how important it was to get inventorship right, particularly in the USA. Mr Ohlson replied saying: "I TOTALLY understand and the inventorship and ideas behind it came from you. Frankly, it's a lot easier that way too. Neil will be fine- and if he's not that's his problem not ours. I will get the form prepared for you." Dr Hill responded thanking Mr Ohlson for his support and saying (about Dr Porter):
"I thought that patent and grant preparation was why we were employing him (at a wage greater than his top consultancy fee rate) so I don't understand this need for 'special appreciation' at having done the job. Yes he worked hard and did a thorough job but that does not warrent falsifying inventorship rights. I guess I am somewhat surprised at this, as he knew what crap I suffered at the RHUL, with this type of issue and I'd have thought he would realise its not a good idea repeating such nonsense as I won't put up with it. He does lose the plot at times!! Oh well time to forget and move on."
188. In fact on Monday 9 March 2009 Dr Hill offered her resignation as an employee to the board of TGL, raising a whole raft of grievances. Her resignation was accepted, to her surprise, by the board at a meeting on 3 April 2009. The events surrounding that resignation are principally relevant to the estoppel case, but it is relevant to Dr Hill's submissions relating to the inventorship dispute to appreciate that the relationship between Dr Hill and Mr Ohlson and Dr Porter, which had been strained for a while, was particularly difficult at around this time. There was a meeting between Mr Ohlson and Dr Hill, together with Mr Lewis (one of the other directors of TGL) on 24 March 2009 to discuss Dr Hill's grievances, at which Dr Hill made it clear that inventorship was a big issue for her and that she was angry that Dr Porter felt he may have a right to be named as an inventor.
189. Mr Ohlson arranged for Dr Porter to speak to Kemps about the inventorship issue. There was an email exchange between Mr Ohlson and two of the other directors of TGL (Mr Lewis and Dr Shafir) on 25 March 2009 as to whether that was necessary or a good idea in the circumstances. However, Dr Porter met Kemps at a meeting on 26 March 2009.
190. On 27 March 2009 Mr Woods emailed Mr Ohlson to say that they would provide a note with their [xxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxxx xxxxxxxx xxxx] in draft form early the next week. On 2 April 2009 Mr Woods sent the draft to Mr Ohlson, asking whether it met his requirements and offering to discuss any changes he would like. On 23 April 2009 Mr Woods chased Mr Ohlson for any comments. The finalised version (which appears to be identical to the draft) was sent to Mr Ohlson on 11 May 2009.
191. Kemps' letter of 11 May 2009 [xxxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxx x xxxxxxx xxxxxx xxxxxxxx xxxxxx xxxxxx xxxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxxx xxxxxxxxx xxxxxxxxx xxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxxx xxxxxxx xxxxxxxx xxxxxxxxxx xxxxxxx xxxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxxxx xxxxxxx xxxxxxxxxxxxx]
192. Kemp's letter of 11 May 2009 xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxx]:
"[xxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxx xxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx
xxxxxxx xxxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxxxxxx xxxxxxxxx xxxxxxxx xxxxxxx xxxxxx xxxxxx xxxxx xxxxxxx xxxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx
xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxx xxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxx xxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx"
193. [xxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxxx xxxx]:
"[xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxxxx
xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx
xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxx
xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxx]"
194. On 20 May 2009 Mr Ohlson emailed Kemps' letter to Mr Lewis and Dr Shafir. Dr Lewis responded to say that he expected that Dr Hill would "challenge this to the bitter end". Mr Ohlson replied saying that he thought it was Dr Porter who "discovered [Heinrich] and put 2 + 2 together" (xxxxxxx xxxxxxx xxxxxxx xxxxx) and took that core idea to Dr Hill. He concluded: "So the question we have to ask ourselves is if/when do we break it to Vanessa. As you rightly ask will she take it out on Neil or the company? My guess would be both (if she is still involved with the company then and if we have to disclose it to her)."
195. There was a board meeting of TGL on 7 July 2009, attended by Mr Ohlson, Dr Porter and Dr Hill. The minutes record that Mr Ohlson "undertook to ask [Kemps] to prepare a report regarding the matter of the "inventorship" for the Board." On 10 July 2009 Mr Ohlson emailed Kemps' letter of 11 May 2009 to Dr Hill. Dr Hill responded immediately to say that she did not accept that Dr Porter had any input into inventorship and the work was solely hers. Kemps' letter was formally put before the board at a meeting on 9 November 2009 which Dr Hill did not attend. In response to the board minutes, on 18 December 2009 Dr Hill sent a lengthy letter and attachments to the board rebutting the points made in Kemps' letter.
196. Ultimately Dr Hill was named as sole inventor on the PCT application and in these proceedings Touchlight does not dispute that Dr Hill was the sole inventor of the inventions disclosed in the priority application and the PCT application, and hence of the Close-Ended Process and the dbDNA Template Process.
197. Mr Cuddigan submitted that Mr Ohlson's conduct relating to the inventorship issue was "extraordinary". He submitted that it was "contrary to one of the principles of natural justice" for Mr Ohlson to arrange for Kemps to speak to Dr Porter but not Dr Hill about inventorship. In cross-examination he put to Mr Ohlson that the procedure was "designed to secure an outcome in which Kemps provide an advice that Dr Porter is a co-inventor" and suggested that it was "some sort of revenge thing". He also suggested that Mr Ohlson was engaged in a "subterfuge" in telling Dr Hill and the rest of the board on 7 July 2009 that he would ask Kemps to prepare a report when in fact they had already done so (which would have been an inept subterfuge given that he then sent Kemps' letter to Dr Hill on 10 July).
198. Mr Ohlson did not accept any of that and neither do I. It is plain that Mr Ohlson was trying to manage a difficult situation, in which it was necessary to come to a decision in the best interests of Touchlight as to who should be named as an inventor in circumstances where there were competing claims from Dr Hill and Dr Porter, Dr Hill had resigned as CSO citing numerous grievances, and the relationship between Dr Hill, Mr Ohlson and Dr Porter was under great strain. It is clear that Mr Ohlson was deeply frustrated with Dr Hill and saw advantages to her resignation. However, I accept that Mr Ohlson had no interest in supporting Dr Porter's claim to inventorship - he just wanted to get things right for the benefit of TGL. Further, the allegation of a breach of natural justice was misplaced. Kemps were not adjudicating a dispute of inventorship between Dr Hill and Dr Porter. They were being asked to advise on the basis of the information they had, as set out in their letter.
199. As mentioned above, Mr Cuddigan also relied on these events as being the reason why Dr Hill was "unable to give her evidence in a dispassionate and objective manner". It is plain that the grievances that Dr Hill had when she resigned as CSO of TGL were increased by seeing Kemps' letter of 11 May 2009 - that much is clear from her response to the board of 18 December 2009. It is also apparent that Dr Hill's sense of grievance against TGL has not significantly been dissipated by the passage of time - as Mr Cuddigan put it, Dr Hill has "been nursing her grievances since then". But if Mr Cuddigan was suggesting that Dr Hill's evidence in this case - and not only the manner in which it was given but also its substance - can be explained and indeed excused by the way in which the issue of inventorship was handled in 2009, then I do not accept that.
200. The more substantive submission on behalf of Dr Hill arising out of the events related above concerned the veracity of Dr Porter's claims to inventorship.
201. In cross-examination, Dr Porter was asked about the contents of Kemps' letter of 11 May 2009. He said he was unable to remember what he had said to Kemps at the meeting on 26 March 2009. When asked about the part of Kemps' letter that addressed the [xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx], he readily accepted that the idea of use of thermophilic conditions and proteins for the DNA amplification step had been Dr Hill's. When asked about what Kemps' letter said regarding [xxxxxxxxxxxxxxxxxxxxxxx], he did not seek to maintain that he had conceived of the Close-Ended Process, but he did maintain that he had conceived of the dbDNA Template Process (xxxxxxxxx xxxxxxxxx xxxxxxxxx xxxxxxx xxxxxxxxxx) - that, of course, is inconsistent with Touchlight's position in these proceedings.
202. Dr Hill submitted that Dr Porter must have provided a false account to Kemps on 26 March 2009. Touchlight submitted that he had merely been labouring under the misapprehension that identifying something as being patentable meant that he was an inventor. But Touchlight also accepted that Kemps would not have been labouring under that misapprehension. Further, it is apparent from Kemps' letter that they [xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxx xxxxxx]
203. In my judgment the likelihood is that Dr Porter did indeed exaggerate his contribution when he spoke to Kemps on 26 March 2009 and claim to have conceived ideas that had in fact been conceived by Dr Hill. [xxxxx xxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxx] Further, the relationship between Dr Hill and Dr Porter was poor at that time, and it is entirely credible that Dr Porter would have wanted to claim credit for ideas that had come from Dr Hill.
204. Dr Hill suggested that if Dr Porter was wrongly claiming credit for inventions when he met Kemps on 26 March 2009, then one could also not trust what he said to Dr Nicholls on the calls in early November 2008. I agree that I should treat any claim by Dr Porter on those calls to have come up with the inventions with a degree of scepticism. But Dr Nicholls' notes do not record any such claim being made - the most one could say is that in the note of the 5 November call Dr Nicholls records Dr Porter as referring to "his" review of the mechanism of action of protelomerase having led to the idea. But the importance of the calls of 5 and 12 November lies not in the light they shed on who made the inventions, but on when the inventions were made.
205. I also need to consider whether my conclusions about Dr Porter wrongly claiming credit for inventions when he spoke to Kemps in March 2009 has any bearing on the reliability of his evidence in these proceedings which bears on the Timing Issue. The amount of evidence which Dr Porter was able to give on matters which related to the Timing Issue which were neither uncontroversial nor apparent from the documents was limited given the effect of the passage of time on his memory. Further, many of the important aspects of Dr Hill's evidence were not actually put to Dr Porter, and I do not believe that it was put to Dr Porter that he was not telling the truth in the evidence he gave in these proceedings. Therefore, I do not regard Dr Porter's exaggerations to Kemps in March 2009 as meaning that I should treat his evidence in these proceedings as being unreliable generally. That is not to say that I accept his evidence that he came up with the dbDNA Template Process, on which I was not at all convinced. But I do not need to reach a conclusion on that question - the question for me to decide is when the dbDNA Template Process was conceived.
206. Finally, some reliance was placed by Dr Hill on an affidavit that Dr Porter signed on 14 September 2018. That contained the following passage:
"Subsequent to September 2008, difficulties were encountered on the research side in isolating suitable thermophilic enzymes for use in the planned process. This prompted a re-evaluation both of patent strategy and of the nature of the process to be commercialised by Touchlight. Further to a review meeting on 27 October 2008 together with Dr Hill and J A Kemp, it was decided to pursue a separate patent application in relation to an in vitro process of DNA manufacture carried out at high temperature, to allow for more time for successful exemplification of use of thermophilic enzymes. As regards protection around the planned closed linear DNA product, a more detailed re-evaluation was needed as regards the possible scope for patent claims. Likely difficulties for patentability of product per se claims to closed linear DNA expression cassettes lacking bacterial vector sequences were discussed at the review meeting. This led us to instead consider protection of a process for production of closed linear DNA.
In the period subsequent to the review meeting on 27 October 2008, an idea was developed of commercialising a wholly enzymatic in vitro process for production of closed linear DNA. This process would combine use of a DNA polymerase and a protelomerase enzyme in an in vitro, cell-free environment, allowing for amplified DNA to be directly converted to closed linear DNA without need for bacterial propagation of DNA. The process could also be carried out with known enzymes, without use of high temperature, allowing us to exemplify the process more rapidly. It also appeared that such a process had not been described previously in the prior art and could thus provide a basis for patentable process claims, avoiding difficulties around claiming closed linear DNA products per se.
I do not specifically recollect the exact date on which the above concept of an in vitro, cell-free process for production of closed linear DNA was first discussed at Touchlight Genetics, but I believe it clearly arose subsequent to the review meeting of 27 October 2008, further to which a significant re-evaluation was needed in relation to patent strategy for protection of our planned closed linear DNA technology. I also recall discussions with Dr James Nicholls of J A Kemp during November 2008 of a particular plan to combine use of the Rolling Circle Amplification (RCA) DNA polymerase phi29 together with a protelomerase in an in vitro cell-free process in which protelomerase would resolve concatamers of amplified DNA created by RCA into single closed linear DNA units."
207. The only aspect of this which was put to Dr Porter as being incorrect was the first sentence. It was suggested that there were in fact no such difficulties, but Dr Porter explained that he meant that no suitable thermophilic enzymes were available. The other point put to Dr Porter was that his affidavit did not say that the Close-Ended Process was his idea following a review of Heinrich, as he had said to Kemps in March 2009. But it does not assist Dr Hill to point out that in his 2018 affidavit Dr Porter did not make the exaggerated claim that he made to Kemps in March 2009 and not to take issue with the rest of the affidavit, which was consistent with Touchlight's case.
208. I need to decide whether, on the balance of probabilities, the Close-Ended Process and the dbDNA Template Process were conceived of before or after Dr Hill's service agreement took effect in early September 2008. I need to decide that taking into account all the relevant documents and the evidence from the witnesses that bears on that question, to which I have referred above.
209. In my judgment, taking everything into account it is more likely than not that the Close-Ended Process and the dbDNA Template Process were conceived after early September 2008, specifically in early November 2008. Indeed, in my judgment that is highly likely to have been the case.
210. My reasons are as follows:
i) There is no credible evidence to support Dr Hill's pleaded case that she conceived of the inventions in March / April 2007. That case relied entirely on her evidence, which is now accepted not to be credible.
ii) There is no basis to think that Dr Hill (or Dr Porter) was even aware of protelomerases and their action on DNA until Dr Hill sent the Ravin email to Dr Porter on 20 November 2007.
iii) Before that time, Dr Hill's idea for preventing concatemerisation of DNA expression cassettes in vivo was to add single-stranded DNA, as shown in her handwritten notes of late October 2007 and recorded in Dr Porter's Stage 1 Report, on which Dr Hill made no comment.
iv) In order for Dr Hill's email of 7 November 2008 (which is the principal documentary evidence in support of her case) to be true, the Close-Ended Process needs to have been conceived by 10 December 2007 (the date on which the Technical Plan referred to in that email was first sent).
v) Neither the Technical Plan created by Dr Hill following her discovery of Ravin and sent to Dr Porter on 20 November 2007, nor the further Technical Plan sent on 10 December 2007, nor indeed any of the iterations of the expanded Technical Plan sent in late December 2007 and early January 2008 describe or show the Close-Ended Process. Dr Hill's case was that they must have had the Close-Ended Process in mind because of the absence of any references in those documents, or the Work Plan, to thermophilic restriction enzymes or ligases. But that is not surprising because the need for such enzymes would only arise if a thermophilic protelomerase was discovered in the future, and that was very uncertain.
vi) The Technical Plan of 26 November 2007 does contain a diagram showing the proposal for producing closed linear DNA using protelomerase (not called doggybone DNA at that time). While Fig. 2 shows signs of being produced in a hurry and/or without being fully thought through, if Dr Hill had by then had the idea of applying protelomerase directly to concatamers, Fig. 2 would not look like it does. The representation of a circular substrate for the protelomerase is inexplicable unless Dr Hill's idea involved such a substrate. Further, the suggestion that Fig. 2 shows (a) the production of the RCA template and (b) what happens in nature is simply not credible. Rather, Fig. 2 is entirely consistent with the proposed process for producing the close-ended DNA expression cassettes involving cutting with restriction enzymes, ligating to form circularised DNA and then applying the protelomerase.
vii) If Dr Hill had conceived of the Close-Ended Process or the dbDNA Template Process by the time of the meetings with Kemps in May to July 2008 it is inconceivable that she would not have explained them to Kemps. The processes are not difficult to explain and illustrate (as the slide decks of November 2008 and 2009 show) or for someone like Dr Nicholls to understand (as his notes show). However, Dr Hill's evidence about communicating the inventions to Kemps is not credible.
viii) The fact that the Close-Ended Process does not appear in the draft patent application circulated in early September 2008 is strong evidence that it had not been communicated to Kemps by that date. The suggestion that the Close-Ended Process can be deduced from Document A (and in particular the skeleton claims) relies on an over-linguistic analysis of a document which was not produced with the purpose of being subjected to such analysis. Dr Hill's email of 5 June 2008 provides (at best) only slight support for Dr Hill's case, when taken in isolation. Further, if the Close-Ended Process had been communicated to Kemps before Document A was produced in June 2008, that would require both Dr Nicholls and Mr Woods to have forgotten about it when drafting the patent application. That is inherently incredible.
ix) No credible explanation has been advanced for why Dr Hill would have responded in the way she is recorded as having done in the meeting with Kemps on 27 October 2008 when asked whether the substrate for protelomerase needed to be circular, if she had conceived of the Close-Ended Process by that time. I do not accept her evidence that she was not aware of the contents of the draft patent application before that meeting; even if she had not been that would not explain her response.
x) Dr Nicholls' notes of the calls with Dr Porter on 5 and 12 November 2008 are entirely consistent with his evidence that he had not been told about the Close-Ended Process or the dbDNA Template Process before those dates.
xi) Dr Hill did not produce any document showing or describing the Close-Ended Process until November 2008. In her August 2008 slide deck the production process was described and shown as involving two steps after the RCA amplification - cutting the concatemers with thermophilic restriction enzymes and then treating the single units with protelomerase to produce the doggybone cassettes. There is no credible explanation for this if in fact Dr Hill had the Close-Ended Process in mind at this time.
xii) The contrast between these slides in the August 2008 slide deck and the comparable ones in the November 2008 and 2009 slide decks (where the process was described and shown as being a two-step process with the protelomerase being applied directly to the concatemers) is stark and powerful evidence in support of Dr Hill's thinking having changed between August and November 2008. The slides in the later slide decks also show how easy it would be to describe and show the Close-Ended Process once it has been conceived. It is not credible that Dr Hill would not have produced slides of the type in the later slide decks sooner if she had conceived of the Close-Ended Process before then.
xiii) In my judgment the evidence clearly points to the Close-Ended Process and the dbDNA Template Process having been conceived in early November 2008. Dr Hill's email of 7 November 2008 is to be explained by her being embarrassed at having missed the inventions and attempting to rewrite history.
211. I therefore determine the Timing Issue in favour of Touchlight. That means that it is strictly unnecessary to address the Contract Issue. However, as the matter was fully argued, and as I have formed a clear view on the issue, I shall do so.
212. It was common ground that I need to approach the Contract Issue on the footing that Dr Hill has won on the Timing Issue, because the Close-Ended Process and the dbDNA Template Process had been conceived and communicated to TGL before the Service Agreement came into effect. As neither party suggested that the inventions were conceived in the period between the Service Agreement being signed and coming into effect, I shall approach the issue on the basis that they were conceived and communicated before the Service Agreement was signed.
213. Dr Hill referred me to the judgments of the Supreme Court in Rainy Sky SA v Kookmin Bank [2011] 1 WLR 2900, Arnold v Britton [2015] AC 1619 and Wood v Capita Insurance Services Ltd [2017] AC 1173, and in particular paragraphs 15-21 of the judgment of Lord Neuberger in Arnold v Britton. Touchlight set out a number of propositions of law by reference to those authorities and ABC Electrification Ltd v Network Rail Infrastructure Ltd [2020] EWCA Civ 1645. I did not understand any of those propositions to be in dispute. The most relevant of them were as follows:
i) The court's task is to ascertain the objective meaning of the language which the parties have chosen in which to express their agreement.
ii) The court does so by focussing on the meaning of the relevant words in their documentary, factual and commercial context. That meaning has to be assessed in the light of (1) the natural and ordinary meaning of the clause; (2) any other relevant provisions of the contract; (3) the overall purpose of the clause and the contract; (4) the facts and circumstances known to or assumed by the parties at the time that the document was executed; and (5) commercial common sense.
iii) The court should disregard subjective evidence of any party's intentions.
iv) In striking a balance between the indications given by the language and the implications of the competing constructions, the court must consider the quality of drafting of the clause.
v) Textualism and conceptualism are tools available to the court to ascertain the objective meaning of the language which the parties have chosen to express their agreement. The extent to which each tool will assist the court in its task will vary according to the circumstances of the particular agreement.
vi) Where there are rival meanings, the court is entitled to prefer the construction which is consistent with business common sense and to reject the other.
vii) Commercial common sense is not to be invoked retrospectively. It is relevant only to the extent of how matters would or could have been perceived by the parties, or by a reasonable person in the position of the parties, as at the date of the contract. The court must be alive to the possibility that one party may have agreed to something which with hindsight did not serve its interest.
viii) When interpreting a contractual provision, the court can only take into account facts or circumstances which existed at the time the contract was made, and which were known or reasonably available to both parties.
214. The Service Agreement was entered into on 8 July 2008, between Dr Hill ("the Director") and TGL ("the Company"). The Effective Date was the date on which TGL had received not less than £300,000 from investors by way of subscription for new ordinary shares (which, as I have said, it is common ground occurred at some point in early September 2008).
215. The clause of the Service Agreement which assigns rights from Dr Hill to TGL is clause 11.2:
"[Dr Hill] acknowledges that [TGL] is the sole owner of any and all Intellectual Property Rights and insofar as any of the Intellectual Property Rights are not vested in [TGL] and in consideration of the salary payable to [Dr Hill] under the terms of this Agreement, [Dr Hill] assigns to [TGL] with full title guarantee, the entire copyright (including future copyright) and all other rights and interests of whatsoever nature in and to the Intellectual Property Rights and any products of the Employment together with the right to take proceedings and recover damages and obtain all other remedies for past infringements in respect thereof throughout the world for the full period of copyright (and of any analogous rights) and all revivals renewals extensions and novations thereof and thereafter (so far as possible) in perpetuity, together with the rights to the same in any manner and through any media as [TGL] shall in its absolute discretion decide."
216. Intellectual Property Rights are defined as follows:
"all rights in or arising from industrial and intellectual property rights including without limitation patents trade marks and/or service marks (whether registered or unregistered) registered designs unregistered designs copyright and database right and rights of a similar nature by whatever name they are known in any country of the world together with any applications for any of the foregoing in any part of the world and the copyright in all drawings plans specifications designs and computer software and all know-how and confidential information created by [Dr Hill] in the course of the Employment together with all such rights, applications, copyright, know-how and confidential information relating to the Projects owned or created by or in the knowledge of [Dr Hill] prior to the commencement of the Employment".
217. In turn, Projects are defined as follows:
"the projects based on the use of thermophilic bacteria described in the Information Memorandum issued by [TGL] in April 2008 and any other projects which [TGL] and [Dr Hill] agree should be included within this definition".
218. In addition the parties referred to certain other clauses. Clause 8.1 provided, in summary, that Dr Hill should not be employed by any other undertaking during the course of her Employment, but clause 8.2 provided that clause 8.1 did not apply, inter alia:
"to [Dr Hill] continuing to be involved in academic research in relation to matters other than the Projects, provided that this does not prevent her from properly discharging her duties as required under this Agreement".
219. Clause 11.1 provided that:
"[Dr Hill] acknowledges that she is in a position of special responsibility and under a special obligation to further the interests of [TGL]. Accordingly any discovery, invention, secret process or improvement in procedure discovered, invented, developed or devised by [Dr Hill] during the Employment (and whether or not in conjunction with a third party) and in the course of [Dr Hill's] duties affecting or relating to the Projects or (subject to Clause 11.7) otherwise relating to the business of [TGL] or any other Group Company or capable of being used or adapted for use in it, shall immediately be disclosed by [Dr Hill] to [TGL] and subject to such rights as [Dr Hill] may have under the Patents Acts 1977 and 2000 will belong to and be the absolute property of [TGL] and shall not be disclosed to any other person, firm or company without the prior written consent of [TGL]."
220. Clause 11.7 provided that:
"It is accepted that [Dr Hill] will continue to be involved (outside the course of the Employment) in academic research in relation to matters other than the Projects and it is agreed that any intellectual property rights arising in relation to such research will belong to [Dr Hill]."
It then went on to place obligations on Dr Hill regarding steps she needed to take before entering into negotiations with a third party relating to commercial development of any such research.
221. As will be seen, the definition of Projects in the Service Agreement refers to "the projects based on the use of thermophilic bacteria described in the Information Memorandum issued by [TGL] in April 2008". It was common ground that this was intended to be a reference to the Information Memorandum issued by TGL in May 2008 (which superseded the document dated April 2008).
222. The parties directed me to the following passages of the Information Memorandum. First, under the heading "The Funding Requirement" it states:
"Touchlight is seeking funding of £1.8 million over the course of the next three years to develop the company's existing knowledge, to secure that knowledge in the form of patented intellectual property and to exploit its significant commercial value. The two specific projects relate to:-
(1) Novel gene based treatments for bacterial infection.
(2) Novel processes for the rapid production of superior DNA vaccines.
It is anticipated that up to £900,000 will be raised at this stage and a further £900,000 in approximately 18 months time once the patent application process is well advanced."
223. Secondly, under the heading "The Touchlight Solution" it says:
"Touchlight has identified two projects with significant need and market opportunity to generate valuable IP. The projects share unique technology based on the use of thermophilic bacteria, hitherto an unexplored branch of genetic science. The technology is described in further detail in Appendices 8 and 9."
"Thermophilic bacteria" is defined in the Glossary as "any bacterial species capable of growth above 45°C up to 300°C." Appendix 8 is a high level diagrammatic explanation of vaccines, DNA vaccines and vector-free DNA vaccines. Appendix 9 is a high-level description of the ThermoLethal Vector approach.
224. The Information Memorandum then further describes the two projects as follows:
"Project 1: Novel gene based treatments for bacterial infection
This will be the lead project and will commence as soon as laboratory facilities have been established. The plan indicates a provisional start date in April 2008. The company has coined the term 'ThermoLethal Vectors' (TLV) to describe the technology (Appendix 9). Two "warhead designs" have been identified and will be genetically designed, constructed and evaluated in parallel. One or both of the warheads will then be further developed and optimised to improve the bacterial lethality properties of the TLV.
Project 2: Novel processes for the rapid production of superior DNA vaccines
This project will begin three months after Project 1 to allow the research to be firmly established and give time for personnel recruitment. The project will screen for 2 new proteins that will form part of a novel and rapid process for manufacturing DNA vaccines and gene therapy treatments."
225. The parties each drew my attention to several aspects of the factual matrix which they said supported their cases. Some of the facts relied on were undisputed (though there was a dispute as to whether they had any, or any significant, relevance to the issue of construction of the Service Agreement) whereas other matters relied on were disputed as a matter of fact as well as of significance. Below I set out my findings as to the factual matrix as of 8 July 2008.
226. First, TGL was a commercial venture established with the principal aim of making money for the benefit of the shareholders, including Dr Hill, who held 49% of the shares. Dr Hill was in particular need of money at the time for personal reasons and to help fund her work on Nature's Code.
227. Second, TGL's initial intellectual capital was to be ideas that had been conceived by Dr Hill.
228. Third, that initial intellectual capital was being used, in the Information Memorandum, to attract investment from third parties.
229. Fourth, it was regarded as critical to TGL that it could obtain patent protection, both to attract investment and to protect the company against competition in its exploitation of Dr Hill's ideas.
230. Fifth, it was recognised that TGL would need the freedom to conduct research and development, and ultimately produce and market products, based on its initial intellectual capital. That was reflected in the Heads of Terms signed in 2007, which at that point envisaged that objective being achieved by a licence agreement:
"A licence agreement will need to be put in place between Dr Hill and TGL giving TGL the right to develop, trial and ultimately bring to market the products derived from the lines of research on which Dr Hill has been working."
231. Sixth, Dr Hill was concerned that her work on Nature's Code should not be covered by any agreement with TGL. She specifically expressed that concern at a meeting with Mr Ohlson and his lawyer, Mr Turnbull of Bircham Dyson Bell, on 8 April 2008. Dr Hill also suggested that it was agreed, before she signed the Service Agreement, that the assignment should cover only thermophilic aspects of her work and not extend to mesophilic aspects. Mr Ohlson rejected that, saying that there was no discussion of such a distinction at the time and that Dr Hill only raised it in 2012. I accept Mr Ohlson's evidence.
232. Seventh, the parties were aware that Dr Hill had previously worked at RHUL. However, I accept Mr Ohlson's evidence that his understanding, based on what Dr Hill had told him, was that she had "taken ownership" of her work done at RHUL (as recorded in his email of 14 October 2007).
233. Eighth, both Dr Hill and TGL were aware of the nature of the proposed projects and the assessments of their viability and patentability set out in the Stage 1 Report, the Technical Plan, the Stages 2-3 Report, the further Technical Plan, the various iterations of the Expanded Technical Plan, the Work Plan, the drafts of the Business Plan, the Investor Presentation, Document A and Kemps' note of the meeting of 13 June 2008.
234. By clause 11.2 of the Service Agreement, Dr Hill assigned to TGL all "rights and interests of whatsoever nature in and to the Intellectual Property Rights". The Intellectual Property Rights were, as can be seen from paragraph 216 above, broadly defined and included "all such rights, applications, copyright, know-how and confidential information relating to the Projects owned or created by or in the knowledge of [Dr Hill] prior to the commencement of the Employment". The question concerns the scope of the phrase "relating to the Projects" and in particular whether that scope is such that it includes the Close-Ended Process and/or the dbDNA Template Process when operated under conditions that are not wholly thermophilic.
235. The limitation to processes operated under wholly thermophilic conditions is said by Dr Hill to arise from the definition of Projects as "the projects based on the use of thermophilic bacteria described in the Information Memorandum" and from the description of those projects in the Information Memorandum.
236. However, the language of clause 11.2 and the relevant definitions does not itself limit the assignment to rights in processes conducted under wholly thermophilic conditions. Rather it limits the assignment to rights relating to the projects based on the use of thermophilic bacteria described in the Information Memorandum.
237. The Information Memorandum itself described the projects at a high level. They were said to relate to (1) novel gene based treatments for bacterial infection and (2) novel processes for the rapid production of superior DNA vaccines, and to share unique technology based on the use of thermophilic bacteria. As regards the first project, it was said that two "warhead designs" would be genetically designed, constructed and evaluated and that one or both of the warheads would then be further developed and optimised to improve bacterial lethality properties. It was said that the second project would screen for two new proteins that would form part of a novel and rapid process for manufacturing DNA vaccines and gene therapy treatments.
238. There is nothing in the description of the projects in the Information Memorandum which limits them to the use of processes conducted under wholly thermophilic conditions. The fact that the projects may "share unique technology based on the use of thermophilic bacteria" does not mean that all processes involved in those projects will operate under wholly thermophilic conditions.
239. Dr Hill relied on clauses 8.2 and 11.7. She observed that those clauses permitted her to continue to be involved, outside the course of her Employment, "in academic research in relation to matters other than the Projects" and would own all rights arising from that research. I do not see how these clauses assist Dr Hill. They are forward-looking clauses delineating matters after the Service Agreement came into effect. I do not see how they restrict the effect of the assignment of pre-existing rights "relating to" the Projects.
240. Thus in my view the language of the Service Agreement, with its cross-reference to the description of the projects in the Information Memorandum, does not on its face limit the assignment of rights to those in processes operated under wholly thermophilic conditions.
241. As I have said, the factual matrix includes the parties' knowledge of the nature of the proposed projects and the assessment of their viability set out in various documents. Those documents do not limit the DNA vaccine project to processes carried out under wholly thermophilic conditions, nor do they show that (as Dr Hill submitted) by July 2008 a wholly thermophilic approach had been adopted for that project. On the contrary, they recognise that while it would be desirable to be able to operate under wholly thermophilic conditions, that might not prove to be possible. For example:
i) In the Technical Plan the section on addition on telomeric ends envisaged the use of protelomerase from phage N15 and its recognition site. It was proposed to "investigate the potential of obtaining a thermophilic version to produce a fully thermophilic process" - see paragraph 76 above.
ii) The further Technical Plan likewise involved the use of N15 protelomerase as well as screening for a thermophilic protelomerase - see paragraph 98 above.
iii) Initial drafts of the Expanded Technical Plan indicated that database searches for a thermophilic protelomerase had been unsuccessful and that screening could take some time - see paragraph 102 above. In a later draft (see paragraph 104 above) Dr Hill added that she was "confident after a brief literature search that there are a number of possible candidate phages that may well carry a version of this gene" but that was far from a guarantee of success.
iv) The Work Plan also showed the project as involving the use of N15 protelomerase, leading to a patent application, with a separate work stream of screening for a thermophilic protelomerase - see paragraph 105 above.
v) The first draft of the Business Plan dated January 2008 identified a number of opportunities to improve process efficiency and generate intellectual property, including "develop a high temperature process for faster process reactions", "screen, isolate and test new high temperature enzymes and proteins to improve process efficiency" and "screen, isolate and test new high temperature enzymes to create improved vector constructs for gene delivery and expression" (see paragraph 106 above). It also referred to Dr Hill's experience with thermophilic bacteria. But it did not indicate that the project was limited to processes in which all steps were carried out under thermophilic conditions.
vi) The Investor Presentation of May 2008 envisaged a fully thermophilic process using a thermophilic protelomerase, but also acknowledged that no such enzymes had been reported to date - see paragraph 108 above.
242. These documents also show that the parties were aware that the protelomerase aspects of the process could be carried out either under thermophilic conditions (assuming a suitable thermophilic protelomerase could be obtained) or under mesophilic conditions. So, on the assumption that the Close-Ended Process and the dbDNA Template Process had been conceived and communicated before the Service Agreement was signed, they show that the parties were aware that those processes (being based on the use of protelomerase) could be operated under both mesophilic and thermophilic conditions. In other words, the Close-Ended Process and the dbDNA Template Process are temperature-agnostic, in that the concept behind each is unrelated to the temperature at which the process is operated, and the parties were aware of that.
243. Dr Hill relied on the advice given by Kemps on 13 June 2008 after the receipt of the landscape searches. Kemps' notes of the meeting say this about patentability of the DNA vaccine process:
"xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx
xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxx xxxxxx
xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx
xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxx"
244. While this advice does recognise that the [xxxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxxx xxxxxxx xxxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx]. Further, it was preliminary advice rather than final definitive advice. I do not agree with Dr Hill's submission that as at the date of the Service Agreement it was the parties' objective expectation that the use of mesophilic conditions in the DNA vaccine project could not be patentable. In any event, the idea of using a protelomerase to produce a DNA vaccine (including, on the hypothesis on which I am working, using the Close-Ended Process and/or the dbDNA Template Process) would be confidential information belonging to Dr Hill (disclosed under the NDA, which gave TGL no right to use it).
245. As indicated above, at the date of the Service Agreement it was envisaged that TGL would be carrying out work using N15 protelomerase while also screening for a thermophilic protelomerase. Dr Hill sought to characterise this as merely "proof of principle" work and relied on Prof. Wittig's agreement with that characterisation. In one sense it was, in that it would provide a good foundation for work with a thermophilic protelomerase if one were to be obtained. But that does not mean that it was not part of the project.
246. Further, it was recognised that the protelomerase aspects of the project had potential to give rise to intellectual property rights, and that a thermophilic protelomerase might prove elusive. If the Service Agreement did not assign rights in any protelomerase-related inventions to TGL, on what basis could TGL safely develop its process using N15 protelomerase? Dr Hill submitted that this was not a problem as there was no risk of a patent which extended to the use of protelomerase under mesophilic conditions, but I reject this for the reasons explained above.
247. Dr Hill also suggested that this was not an issue because the only purpose of the Service Agreement was to assign to TGL rights which it could use to obtain patent protection. I do not agree. The purpose plainly also extended to assigning rights which TGL could use to develop the processes forming part of the Projects.
248. Finally, there was some suggestion on behalf of Dr Hill that this problem could be addressed by an implied licence. Originally a licence agreement was contemplated in the Heads of Terms (see paragraph 230 above). But the mechanism of an assignment in the Service Agreement was adopted instead. Dr Hill's suggestion amounts to saying that it would be necessary for business efficacy to imply a licence into the Service Agreement to fill the hole left by the limited (on her case) assignment. In my view the fact that there would be such a hole which would need filling tells against Dr Hill's proposed construction of the Service Agreement.
249. Overall, my task is to ascertain the objective meaning of the language which the parties have chosen in which to express their agreement, by focussing on the meaning of the relevant words in their documentary, factual and commercial context, having regard to (1) the natural and ordinary meaning of the clause; (2) any other relevant provisions of the contract; (3) the overall purpose of the clause and the contract; (4) the facts and circumstances known to or assumed by the parties at the time that the document was executed; (5) commercial common sense.
250. The natural and ordinary meaning of the clause is that it assigns to TGL all Dr Hill's rights relating to the Projects, being those based on the use of thermophilic bacteria described in the Information Memorandum. Neither the Service Agreement itself nor the description of the projects in the Information Memorandum limit those rights to ones in processes carried out under wholly thermophilic conditions. If an invention relates to the Projects but is temperature-agnostic, then it is covered by the natural and ordinary meaning of the relevant clause. There are no other provisions of the Service Agreement which suggest otherwise.
251. The overall purpose of the clause and the Service Agreement, in so far as relevant, was to ensure that TGL had the rights which it needed to take the Projects forward, including, but not limited to, rights which could form the basis of patent protection. The parties knew at the time that the Service Agreement was entered into that the DNA vaccine project involved the use of protelomerase in a process (including, on the assumption on which I am operating, the Close-Ended Process and the dbDNA Template Process) which could be operated under mesophilic or thermophilic conditions and which could give rise to intellectual property rights. They also knew that while a wholly thermophilic process was desirable, it might well not be achievable. It would accord with commercial common sense for TGL to hold the rights in such a process, so that it could develop its process using the mesophilic protelomerase, and continue to use it if a suitable thermophilic protelomerase could not be obtained.
252. For these reasons, I agree with Touchlight's construction of the Service Agreement. Rights in the Close-Ended Process and the dbDNA Template Process would (if they had been conceived before the Service Agreement was signed) have been rights relating to the Projects within the meaning of the Service Agreement and so would have been assigned to TGL by Dr Hill.
253. As I have determined both the Timing Issue and the Contract Issue against Dr Hill it is not strictly necessary to address Touchlight's estoppel case. However, both parties urged me to do so in any event.
254. I have considered the extent to which it is appropriate and proportionate for me to address the estoppel case in this judgment. I have in mind, in particular, that Touchlight relied on four different forms of estoppel: estoppel by convention, estoppel by acquiescence, estoppel by representation and proprietary estoppel. It submitted that the principles of each form have considerable overlap but also significant differences; its summary of the principles of each occupied seven pages of its opening skeleton argument. Further, there were disputes between the parties about aspects of the principles of law, in particular but not limited to whether any of the forms of estoppel relied on have permanent or merely suspensory effect.
255. I have come to the conclusion that, given that I have found against Dr Hill on both the Timing Issue and the Contract Issue, it would be unnecessary and disproportionate to address the law in detail and try to resolve the disputes on the law, or to come to a conclusion about whether, on the facts as I find them, one or more of the doctrines relied on by Touchlight would apply and, if so, what effect that would have on the relief sought by Dr Hill. Instead, I propose to make findings of fact, bearing in mind the principles of law articulated by the parties and the disputes about them, so that if this case goes further and it becomes necessary to resolve the issue of estoppel the Court of Appeal can apply the law (having resolved any disputes) to the facts as I have found them.
256. It is convenient to structure my consideration of the facts by reference to the three limbs of any estoppel case which were identified by Touchlight (though without implying that I accept Touchlight's formulation of the legal principles where there is a dispute). It summarised the three limbs as follows:
Limb 1:
- Estoppel by convention: Was there a common assumption as to the rights enjoyed by Touchlight which "crossed the line" between the parties?
- Estoppel by representation / proprietary estoppel: Was there a representation (or implied promise) emanating from Dr Hill (whether by words or conduct, and whether explicit or implicit) as to the rights enjoyed by Touchlight?
- Acquiescence: Did Dr Hill know that Touchlight was acting in the belief that it was entitled to apply for and hold the patents in issue, and did she stand by in silence when she was under a duty to speak up?
Limb 2: Did Touchlight rely upon Dr Hill / did Dr Hill induce Touchlight to alter its position?
Limb 3: Has Touchlight suffered detriment by reason of the matters set out above?
257. It was common ground that I need to approach the estoppel issue on the footing that I am wrong about the Timing Issue and the Contract Issue. In other words, I must proceed on the basis that the objective meaning of the Service Agreement is that it only assigned to TGL rights in the inventions disclosed and claimed in the priority application, the PCT application and the Patents in so far as those processes were operated under wholly thermophilic conditions. However, that does not mean that Touchlight could not have had the subjective view that it was entitled to the whole of the inventions, nor that Dr Hill could not have shared that view and/or conducted herself so as to lead Touchlight to believe that she shared that view.
Events leading up to the filing of the priority application
258. Touchlight relied heavily on Dr Hill's involvement in the events leading up to the filing of the priority application on 30 January 2009.
259. I have considered above whether Dr Hill was aware of the contents of the 5 September 2008 draft patent application before the meeting with Kemps on 27 October. I find that she was sufficiently aware of its contents to appreciate not only that it described the Cut and Ligate Process (and not the Close-Ended Process), but also that it described and claimed a process which was not limited to wholly thermophilic conditions. Certainly, given that Dr Hill had been asked to review the draft and had agreed to do so, TGL was entitled to assume that she had done so.
260. I have referred above to Kemps' handwritten notes of the meeting on 27 October 2008 and Kemps' letter of 3 November 2008 summarising the matters discussed at that meeting. The handwritten notes say this:
"(4) Temperatures
- Only thermophilic step is amplification step b)
- Ensure other steps can be non-thermophilic or thermophilic
- NB doesn't matter if temp is cooled after amplification as SSBPs prevent snap back of unmeshed concatamers - allude to this?"
Similarly the letter says this:
"3. Non-thermophilic steps
As explained in our meeting, claim 1 only requires a thermophilic amplification step. All other process steps can be carried out at any temperature. Also, the claim does not exclude the possibility of initial non-thermophilic and/or PCR-type steps. I will ensure that the description provides a disclosure that all steps other than amplification step b) may be thermophilic or non-thermophilic, and a list of appropriate temperatures."
261. It is also relevant to note that at this stage claim 14 was a product claim, to closed linear DNA having hairpin ends comprising telL and telR sites flanking a central double-stranded region. The handwritten notes record Dr Nicholls advising that [xxxxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxx] (original emphasis). Similarly, the letter advised that "claim 14 does not limit preparation of the product to a particular process; it covers the product made by any process. [x xxxxxx xxxxxx xxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxxx]"
262. It is clear from the handwritten notes that Dr Nicholls explained at the meeting on 27 October that the draft patent application was not limited to processes carried out wholly under thermophilic conditions. As mentioned above, Dr Hill said that she had not read the draft application before the meeting (which I have rejected) and that she was baffled and confused by some of the questions raised. The notes of the meeting indicate that she was engaged (and it appears from Dr Porter's email of 4 November 2008 that she came up with cassette designs at the meeting - see paragraph 163 above). Further, Dr Nicholls' explanation that the draft application was not limited to wholly thermophilic processes was not a question, let alone one which could have baffled and confused Dr Hill. She also suggested that she had been confused because Dr Porter had told her on the way to the meeting that there would be broad-ranging discussions and that nothing was set in stone, but at the meeting she found that everything had been set in stone. In fact, it is clear from the notes of the meeting that things were not set in stone. In any event, that does not grapple with the point, which is about whether she understood Dr Nicholls' explanations. I find that Dr Hill must have understood Dr Nicholls' explanations, but even if she did not, TGL was entitled to assume that she had.
263. In any event, Dr Hill was sent the letter of 3 November which reiterated those explanations. Dr Hill suggested that she had not read it, but it is inherently unlikely that she would not have read such a letter on a topic of great importance to TGL. Further, on 4 November Dr Porter emailed Dr Nicholls to thank him for the meeting summary, saying "I have spoken to Vanessa and Jonny and we agree with your recommendation to separate the product and process claims and submit two separate patent applications. [xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx xxxxxx] As you pointed out, we need to put some additional thought into inventive steps related to the products. We'll work on this and get back to you for opinion." This suggests that Dr Hill had considered the 3 November letter. I hold that she had. In any event, TGL was entitled to assume that she had done so.
264. In any event, it is clear that by 7 November 2008 Dr Hill had considered the draft patent application. She asserted that she had done so before then (though after the meeting of 27 October) and that is clear from her email of 7 November (on which she relies heavily in relation to the Timing Issue) - see paragraph 167 above. She must have appreciated, by then at the latest, that the draft application was not limited to processes operated under wholly thermophilic conditions.
265. Following TGL's agreement to the proposal to submit two separate applications, on 27 November 2008 Dr Nicholls emailed Dr Porter a draft application relating to the improved DNA amplification aspects, together with a covering letter. The letter explained that all subject matter relating to the use of protelomerase had been removed and would be pursued in a separate application, and that the description contained basis for all steps other than the strand displacement step being carried out under non-thermophilic conditions (which indeed it did). Again Dr Hill suggested that she would not have had time to read the letter or the draft application and that she had not done so (at least properly). Again it is inherently unlikely that she would not have done so. Further, it is clear from an email and attachments sent by Dr Porter to Dr Nicholls on 3 December 2008, responding to the letter of 27 November, that she must have done so. For example, in one of the attachments Dr Porter says: "Vanessa has pointed out that, at present, our claims appear to cover only the use of a single DNA polymerase. ..."
266. On 12 December 2008 Dr Nicholls emailed Dr Porter, Dr Hill and Mr Ohlson a finalised version of the same application and a covering letter asking for comments and for agreement to place it on hold pending completion of the second application. Again Dr Hill suggested that she would not have read these documents. This time there is no documentary indication that she did what would have been expected of her, but in any event TGL was entitled to assume that she had.
267. On 18 December 2008 Dr Nicholls emailed Dr Porter, Dr Hill and Mr Ohlson to say that he had been reviewing material provided by Dr Porter (including cassette designs based on Dr Hill's November 2008 slide deck) and suggesting that, to assist drafting and save costs, Dr Porter and Dr Hill might prepare some descriptive text for the second application. Following discussion with Dr Hill, Dr Porter sent a draft to Dr Nicholls on 7 January 2009. Dr Nicholls then sent a draft application entitled "Production of Closed Linear DNA" to Dr Porter, Dr Hill and Mr Ohlson on 16 January 2009, together with a covering letter. The letter explained (original emphasis):
"As suggested, the application is now focussed on a process for production of closed linear DNA that involves use of DNA polymerase and protelomerase in combination. ...
The proposed claims are broadly directed to amplification based on any DNA template comprising a protelomerase target sequence, using any DNA polymerase, and a subsequent processing step for the amplified DNA using any protelomerase. Please let me have your comments on this proposed claim scope. ..."
That was an accurate summary of the accompanying draft application, which contained no temperature limitations and expressly contemplated the use of mesophilic polymerase and protelomerase.
268. At this point it is convenient to mention a refrain that ran through Dr Hill's oral evidence about the patent applications, namely that she repeatedly asked for them to be put on hold because she felt that they were being rushed ahead when they were not ready. There is no evidence in the documents to support that assertion. On the contrary, also on 16 January 2009 Dr Hill emailed Mr Ohlson and Dr Porter a note of "urgent matters" which she felt needed consideration. These matters related to issues with setting up the laboratory. In this context she said: "As we now have nearly 2 patents completed I think we should rest from doing any more here (apart from getting the final draft of 1b [i.e. that relating to production of closed linear DNA]) and clear this backlog of tasks ASAP." This is significant for two reasons. First, Dr Hill was requesting stopping work on patent activity after the two applications being prepared had been filed. Secondly, it indicates that she was being diverted by work on the patent applications, contrary to her evidence that she had not read them.
269. On 20 January 2009 Mr Ohlson emailed Dr Hill a proposed agenda for a TGL directors' meeting on 23 January. Point 1 was "CSO feedback and sign off to patent applications from Jimmy's email dated 16th Jan." The minutes of the meeting on 23 January record the following action points for Dr Hill (with high priority): "1) Complete drafting of patent 1(b) for signing off and filing with patent 1(a) on Friday 30th January 2) Prepare list of questions for NP to raise with Kemps by 27th January 3) Prepare list of experiments to support patents 1(a) and 1(b)".
270. Notwithstanding this, Dr Hill said that she had not read Dr Nicholls' letter of 16 January 2009 nor the accompanying draft application (i.e. "patent 1(b)"). She said that she had no time, that she wanted the patents put on hold and that the decision at the meeting involved overriding her. I do not accept any of that. Further, Dr Nicholls' file note of a call with Dr Porter on 27 January 2009 indicates that Dr Hill had some concerns about [xxxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxxxxxx], so it appears that Dr Hill had been engaging with the draft application.
271. On 28 January 2009 Dr Nicholls emailed Dr Porter, Dr Hill and Mr Ohlson a final draft of the application relating to production of closed linear DNA, asking for final comments on the specification or its approval for filing. As with the draft sent on 16 January, there were no limitations as to temperature.
272. On 29 January 2009 Dr Hill emailed Mr Ohlson saying: "Neil and I have been through the patent this morning and apart from getting the word telomere resolvase into it somewhere all seems fine-very thorough I think-James has done a very good job here."
273. Yet again, Dr Hill said that she had not read this final draft. She suggested that Dr Porter had not actually gone through the draft with her but just explained it to her in a cursory way. She relied on Dr Porter's evidence that it was unlikely that he ran through every page of the document with her on 29 January. However, Dr Porter added that that would have been a waste of time as they had reviewed previous drafts. I reject the suggestion that Dr Hill had not, before and/or at the meeting on 29 January, reviewed the entire draft. That would be completely inconsistent with Dr Hill's 29 January email. In order to express the views that "all seems fine", that it was "very thorough" and that Dr Nicholls had done "a very good job" and to comment that "telomere resolvase" should be inserted, she must have read the draft from beginning to end.
274. Dr Porter passed on Dr Hill's request for the inclusion of "telomere resolvase" to Dr Nicholls on 29 January 2009. There was a further directors' meeting on 30 January 2009 at which it was recorded that tasks 1-3 (see paragraph 269 above) had been completed. The priority application was filed on the same day (as was the application relating to improved DNA amplification).
275. I find that Dr Hill was aware that the priority application contained no limitation as to temperatures at which the process for producing closed linear DNA could be operated, and indeed stated that it could be operated using mesophilic enzymes as well as thermophilic ones. Indeed, she had been aware since at least late October 2008 that the patent applications being drafted by Kemps were not limited to processes operated under wholly thermophilic conditions. Yet at no point did she raise any objection to TGL filing applications of such a nature in its own name. On the contrary, she approved the priority application being filed in TGL's name (at least on 29 January 2009 - Mr Ohlson said he recalled her telling him orally that she was happy with the draft on previous occasions as well).
276. Dr Hill submitted that when she approved the priority application she was doing so as an employee and director of TGL, rather than in her capacity as a counterparty to the Service Agreement. She also submitted that her approval of the priority application could not be taken as a clear indication that filing the priority application in TGL's name was in accordance with the assignment under the Service Agreement, and that she could not be taken as having assumed responsibility for assessing compliance with the Service Agreement.
277. In my view those submissions miss the point. First, the distinction between Dr Hill's role as a counterparty to the Service Agreement and her role as an employee pursuant to the Service Agreement is a rather slender one. But in any event, Dr Hill had duties, as a director and under the Service Agreement, to further the interests of TGL. In my view those duties obliged her to notify TGL if it was filing a patent application for an invention to which, to her knowledge or in her view, it was not wholly entitled, and TGL was entitled to assume that she had complied with that obligation. Secondly, Touchlight is not relying on a representation as to the construction of the Service Agreement, but on a representation that TGL was entitled to file the priority application in its own name.
278. For similar reasons, I do not accept Dr Hill's submission that any representation was one of law, i.e. as to the true construction of the Service Agreement. Dr Hill indicated that she was happy for the priority application to be filed in the name of TGL, without asserting any rights in the inventions which it disclosed and claimed. I do not regard that as merely a representation as to the construction of the Service Agreement, but as being a representation that she claimed no rights in the inventions vis-à-vis TGL.
Events leading up to the acceptance of Dr Hill's resignation
279. By early March 2009 the issue of inventorship had arisen (see above) and on 9 March 2009 Dr Hill tendered her resignation as an employee and CSO of TGL. At least by this point Dr Hill had been receiving advice from the solicitors Dzimitrovicz York. There was a board meeting on 20 March 2009 for which Dr Hill prepared a document outlining her grievances and at which she made an oral statement. The document contained 12 points, of which the fourth was (original emphasis):
"A serious problem is my contract of employment. I explicitly agreed to come on board this venture only if certain criteria were fulfilled. The most important of those was the separation of any rights to my work concerning a new ground breaking theory I am developing with a quantum physicist and any spin-off technologies that may come from them or, indeed, any other ideas that are unrelated to the Touchlight projects. I now find I have a contract which, although prepared by our company lawyer, who was explicitly told by myself my requirements, is actually one that threatens my full IP ownership rights and requires me to take the company to court to prove these ideas do not belong to TLG! I had to resign on the basis of this very fact alone, as I cannot accept the situation at all."
280. The minutes of the meeting record two of Dr Hill's key reasons for her resignation as being:
"(4) She had agreed to transfer her ground breaking ideas in return for a 49% stake in the Company.
(12) She was concerned that her ideas for "Natures Code" had been included in the transfer to the Company and that ideas outside of the specifics referred to in the "Information Memorandum" had also been transferred and that she would have to sue the Company to prove her rights."
281. The board's response to those points is recorded as follows:
"(4) It was agreed that she had transferred her ideas but that this was in connection with the "Projects" as defined in her contract and contained in the Information Memorandum.
(12) The question of Natures Code research had been addressed as it was not within the "Projects" and therefore remained her property."
282. On 2 April 2009 Dr Hill emailed the other members of the board with her proposed amendments to the minutes. She highlighted the words "ideas outside the specifics referred to in the Information Memorandum" in point (12) of the list of her recorded key reasons for resignation and added "?dont understand this-didnt say this". Against point (4) of the board's recorded response she added "Dont remember any reference to an Information Memorandum-where is this?" Dr Hill suggested in her oral evidence that her only objection in relation to point (12) had been about the use of the term "Information Memorandum". While that plainly was part of her objection to what she was recorded as having said, the fact that she also highlighted the words "ideas outside of the specifics referred to in" indicates that her objection went beyond that.
283. Looking at the document which Dr Hill prepared for the meeting, and taking into account the minutes and Dr Hill's comments on them, one can see that her concern as expressed to TGL was that Nature's Code, or any other ideas which were unrelated to the Touchlight projects, might have been assigned to TGL by the Service Agreement. In other words, her expressed concern was that the assignment under the Service Agreement might have been broader than either construction advanced in these proceedings. There is nothing to suggest that she had any issue about ideas which were related to the Touchlight projects having been assigned. Nor is there anything to suggest that she saw a distinction between rights in processes carried out under wholly thermophilic conditions and rights in processes which were not, still less that she communicated that to TGL.
284. Dr Hill submitted that points (4) and (12) in the board's response amounted to a clear formal acknowledgment that the rights which Dr Hill had assigned were only those covered by the Service Agreement. She submitted that the board was agreeing to be bound by the terms of the Service Agreement as they were objectively to be understood and so had renounced its ability to rely on its understanding of the Service Agreement in relation to the point now in issue. I do not agree. The board was indicating its understanding of the position and of the Service Agreement in general terms. The issue of whether the Service Agreement assigned rights in processes only under wholly thermophilic conditions or more generally had not been raised and indeed Dr Hill had approved the filing of the priority application in TGL's name. The board cannot be understood as having indicated that it was abandoning its understanding of the Service Agreement, whatever issue might arise in the future.
285. Following the board meeting, on 24 March 2009 Mr Ohlson and Mr Lewis held a meeting with Dr Hill to discuss the issues which led to her tendering her resignation. The notes of the meeting show that there was discussion on the issue of Dr Hill's "contract (i.e. clarification on IP/ideas, new ideas/reward etc)". They record that she wanted the contract redrafted to make it absolutely clear which of her ideas belonged to TGL (and wanted Nature's Code excluded). On 25 March 2009 she emailed Mr Ohlson a "wish list" which included:
"Contract alterations re Nature's code and any other ideas/technologies that are not directly related to the technology I have brought to TLG. Removal of clauses that say I have to present these other ideas for TLGs consideration first-this should remain at my discretion alone."
286. Again, there is no suggestion that Dr Hill regarded the assignment under the Service Agreement as excluding ideas relating to TGL's projects (her concern was that it might be even more extensive) or that she regarded the rights assigned as being limited to processes conducted under wholly thermophilic conditions.
287. On 3 April 2009 there was a further board meeting at which Dr Hill's resignation as an employee was formally accepted.
Dr Hill's concerns about the direction of TGL following her resignation
288. On 3 July 2009 Dr Hill sent a letter to the board in which she said:
"As I am a Director and the major share holder of [TGL], it is obviously in my interests that the company does well. It is also obvious that I should want to that see that my original, lifetimes work is being furthered in the best manner possible for this company's interests."
She went on to express concern that insufficient work had been done on finding thermophilic enzymes, even though they were "crucial to establishing a full high-temperature process of vaccine manufacture", adding: "As the patent lawyers have advised, [xxxxxx xxxxxx xxxx xxxxx xxxxx xxxxx xxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxx]" The note went on to refer to the work that was being done using mesophilic enzymes. There was no suggestion here, or in a list of points she raised for discussion at the board meeting on 7 July 2009, or in the minutes of that meeting, that Dr Hill regarded that work as being something which TGL was not entitled to undertake.
289. In October 2009 TGL put various settlement proposals to Dr Hill which were rejected and on 9 November 2009 the board resolved to execute a letter of her resignation as a director. On 18 December 2009 Dr Hill sent a lengthy letter to the board regarding inventorship. While asserting that she was the sole inventor of the various inventions (and that Dr Porter was not an inventor) it did not suggest that TGL was not the sole owner of those inventions.
290. On 9 February 2010 Dr Hill sent Mr Ohlson an email from herself and Mr Paul Manweiler, a significant shareholder in TGL (copied to her solicitor Tony Dzimitrowicz), saying:
"Paul and I require further clarification of the present status of the company's two patents (referring to them as patent 1 (part 1 of process) and patent 2 (part 2 of process)) that were filed in January 2009. We need this information before we can come to a decision regarding the purchasing of further shares. Please answer these points as fully as possible.
1. How many of the individual claims have been fully supported by actual hard data both in patents 1 and 2?
2. Has the data been generated to fully support the high temperature aspects of these patents both in patents 1 and 2?
3. Have the patents been withdrawn or are they continuing to international status and therefore will be published in 18 months (approx) time?
4.Why have I, the original inventor, not been requested to sign the inventorship forms?
5. Who has been declared as inventor/s upon patents 1 and 2?"
291. As will be seen, Dr Hill's concerns related to inventorship and to whether the patent applications were being pursued and were to be fully supported by data that had been generated, including for "the high temperature aspects" (which indicates that she was aware that there were also aspects which did not relate to high temperatures).
292. Mr Ohlson responded on 16 February 2010, saying:
"A PCT application for patent 2 entitled "Production of closed linear DNA" has been filed. We believe that all the claims in this patent have been substantiated by scientific data developed between May and December 2009. The patent application covers widest range of physical conditions for carrying out the process including temperature. Our patent attorneys inform us that Dr Vanessa Hill has been declared as the only inventor on the PCT application. The application will be published in six months time (six months from the 31st January 2010)."
He went on to explain that a PCT application had not been filed based on the "DNA amplification" application and that it had not been possible to achieve satisfactory thermophilic DNA amplification.
293. Dr Hill replied to Mr Ohlson in an email dated 19 February 2010, expressing pleasure that she had been named as inventor. After various other comments she concluded:
"In conclusion, 1 presume 1 am correct in saying, that supporting data was only obtained for patent 2 and yet only the mesophilic range of temperature of operation within that patent; Targets were not reached for patent 1 and no data at all obtained for the TLV system. So effectively, supporting data for only 1 third of the proposed patents were obtained and only the lower temperature part supported here. This falls well short (by aprox 80%) of our initial plan. Am I correct in this?"
294. In her oral evidence, Dr Hill said that it was only on receipt of Mr Ohlson's email of 16 February that she knew that the application related to all temperatures. While Dr Hill had not at this point seen the PCT application, for the reasons explained above I reject the suggestion that she was not aware that TGL was filing patent applications which related to all temperatures. In any event, she responded to Mr Ohlson's email by complaining about the fact that "only the mesophilic range of temperature of operation" was supported, rather than about TGL filing a PCT application which covered the "widest range of physical conditions for carrying out the process including temperature". She said that she did not do so as she could not afford patent lawyers. But that had not stopped her making complaints about inventorship, and if she had in fact regarded the scope of the PCT application as something about which she could complain I have no doubt that she would have done so.
295. The PCT application was published on 5 August 2010. Dr Hill's evidence was that she was shocked to see that it was not limited to wholly thermophilic processes. I reject that. Not only was she aware that the priority application had no such limitation, Mr Ohlson had confirmed that the PCT application had no such limitation in his email of 16 February.
296. Having read the PCT application, on 11 August 2010 Dr Hill sent a letter to the board of TGL in which she complained that a number of targets had not been met which she regarded as "crucial to realising the true value of the intellectual property that I, as the inventor, brought to the company in return for my shareholding." Having complained about the abandonment of the ThermoLethal Vector project, she said:
"The second technology also, has been drastically compromised as most of the required, crucial research targets were not realised. As a consequence, one of the patents (GB0901592.6) has been withdrawn and re-submitted (thus losing prior art date) and the second patent (GB0901593.4) has also been seriously compromised by the lack of supporting data. This has meant that Touchlight has not acquired the all important proprietory enzymes and DNA products/methodologies that would have given Touchlight's technology the leading edge over our main competitors. In fact, submission of patent (GB0901593.4) has actually advertised to our competitors a way of obtaining an advantage over our company as it is now published and in the public domain. I also have concerns that with the publication of the patent WO/2007/087478 and other academic publications by these authors, that many of the claims in our own will be lost due to the lack of high temperature counterparts. This situation is drastic and has now greatly reduced the potential efficacy and value of the intellectual property held by Touchlight."
The letter went on to reiterate her complaint about the PCT application containing "no supporting data for high temperature enzyme counterparts" before concluding by expressing the view that her "inventions given to Touchlight in return for my major shareholding, had huge potential to realise considerable commercial value" but that commercial value was in danger of being lost.
297. As is apparent, Dr Hill's complaint was not that the scope of PCT application extended to processes that were not wholly thermophilic. On the contrary, she recognised that the PCT application was the result of the intellectual property that she brought to TGL. Her concern was that the high temperature aspects of it were insufficiently supported. Again she suggested that the absence of any complaint about the scope of the PCT application was due to lack of legal advice. But I reject that for the same reasons as explained in paragraph 294 above.
Subsequent events
298. In late 2011 Kemps sent Dr Hill a number of documents relating to national patent filings stemming from the PCT application, including statements of inventorship and confirmatory assignments. That led to correspondence between Dr Hill and Kemps. In an email of 7 February 2012 to Mr Woods (copied to Mr Lewis) Dr Hill wrote:
"I have now had the opportunity to take advice on our ongoing matters. As you are aware I have already sent documents and emails that appear to raise questions regarding whether the invention in the Touchlight patent application was first conceived prior to me joining Touchlight Genetics Limited. This appears to bring into question the ownership of the invention and the right of Touchlight Genetics Limited to apply for patents for that invention.
It would be good to resolve this matter. As you are aware, all I want from you is the standard assignment from you to me of the Thermolethal Vector Technology (TLV), which has already been provided to you. In return, I'm happy to retrospectively assign any and all rights I own in the invention disclosed in the International Patent Application No PCT/GB2010/000165, subject to the condition below.
It is quite clear that there are questions in relation to ownership of the invention disclosed in that patent application that should be addressed. Consequently, I regard it as only fair and proper that some payment should be made to me to clear these matters up and to move forward. To that end, I suggest a sum of £200,000 given the value of the markets in which you wish to exploit this patent and how this invention is likely to be of outstanding benefit to Touchlight Genetics Limited."
299. Mr Woods responded by a letter dated 1 March 2012 rejecting Dr Hill's suggestion that she owned any rights in patents stemming from the PCT application. Dr Hill replied to Mr Woods by an email dated 8 March 2012, saying:
"As you will be aware, the definition of "Intellectual Property Rights," as laid out in the Service Agreement, is specifically focused on IPR "created by the Director in the course of the Employment together with all such rights, applications, copyright, know-how and confidential information relating to the Projects (my emphasis) owned or created by or in the knowledge of the Director prior to the commencement of the Employment."
This leads naturally to the Service Agreement definition of the "Projects" where it makes explicit reference to "thermophilic bacteria," as described in the Information Memorandum issued by the Company in April 2008. Therefore, any IPR relating specifically to "thermophilic bacteria" in relation to either Project 1 and Project 2 would have been transferred to Touchlight Genetics Ltd (TLG) upon signing the service agreement.
Conversely, the TLG International patent application - WO2010GB00165, claiming priority from GB901593.4 - and national/regional rights derived therefrom are not limited to thermophilic bacteria and are based upon research and ideas undertaken by me prior to joining TLG. It is important to note that while I had written various documents, including two research papers on non-thermophilic technology, this process does not in any way form part of the definition of the "Projects." Therefore, it does not fall within the definition of IPR, as set out in the terms of the Service Agreement."
300. This is the first document in which Dr Hill sought to draw a distinction, in terms of ownership, between thermophilic and mesophilic conditions. I accept Mr Ohlson's evidence that Dr Hill did not seek to make that distinction before March 2012.
301. Again Mr Woods rejected Dr Hill's claim, in an email dated 13 March 2012, to which Dr Hill responded on 14 March. On 4 April 2012 Wellers, solicitors acting for TGL, sent Dr Hill a detailed letter rejecting her claim, to which Dr Hill responded by email on 5 April. From May to December 2012 there were various proposals for settlement by both sides but no agreement was reached.
302. The correspondence then ceased. It was not until 5 January 2021 that Mishcon de Reya wrote their letter saying that Dr Hill "considered the historic IP disputes to remain extant" and not until 21 November 2022 that Wiggin sent Dr Hill's letter of claim. Dr Hill explained that she had not been in a position, for financial and health reasons, to bring a claim for many years.
Assessment
303. In my judgment it is clear that in the period prior to 2012 Dr Hill and TGL shared the view that TGL owned all the rights in the inventions that were disclosed and claimed in the priority application and the PCT application. Mr Ohlson was not challenged on his evidence that that had always been his understanding, which was shared by other members of the board. If Dr Hill had not shared that understanding and instead had believed that she owned rights in processes carried out under mesophilic conditions, it is inconceivable that she would not have raised that point (i) during the process of preparation of the priority application, (ii) in her lists of grievances presented leading up to her resignation being accepted or (iii) in the communications during 2009 and 2010 in which she complained about perceived failures in the direction of TGL. Dr Hill's acts and statements were plainly enough to convey to TGL that she shared its view that it owned all the rights in the inventions. In my judgment there was a common assumption that TGL owned all the rights in those inventions which "crossed the line" between the parties. Similarly, in my judgment Dr Hill's acts and statements in the period prior to 2012 were sufficient to amount a representation that TGL owned all the rights in those inventions.
304. However, Dr Hill's statements in February and March 2012 made it clear to TGL that she no longer shared the view that TGL owned all the rights in the inventions, and instead asserted a claim to those inventions in so far as they were operated under mesophilic conditions.
305. It is possible to take these two limbs together and deal with them rather more briefly than Limb 1.
306. Mr Ohlson explained in his first statement that he understood Dr Hill to be of the view that TGL was entitled to file the priority application, based on her email of 29 January 2009 as well as prior oral statements that she was happy with drafts of the application. He then referred to the minutes of the board meeting of 2 December 2008 (which approved the filing of two applications). He then said that the board would not have permitted TGL to file the priority application if it had understood that Dr Hill had not been happy with the position and that:
"Relying on the understanding and assurance that Dr Hill was satisfied with everything as I have just described, the board permitted [TGL] to finalise and make the Priority Application (as well as the subsequent PCT Application, which resulted in granted patents) and to expend significant time and money developing the business into what it is today."
307. It was put to Mr Ohlson that his evidence of reliance related solely to the board meeting of 2 December 2008. It was then put to him that the only application that had been drafted at that time was the one relating to improved DNA amplification, in which claim 1 related to an amplification process carried out under thermophilic conditions. The suggestion was that therefore the board could not have been relying on any representation by Dr Hill as to TGL's rights in processes which were not carried out under wholly thermophilic conditions.
308. I do not accept that. First, while claim 1 of the draft application relating to improved DNA amplification did specify that the amplification step should be carried out under thermophilic conditions, the description made it clear that other steps, including cutting the concatemers, could be carried out at any temperature. Secondly, Mr Ohlson made it clear that his evidence did not relate solely to what happened at the board meeting on 2 December 2008. Indeed, there was a meeting of the directors of TGL on 30 January 2009, following Dr Hill's email of 29 January, at which it was recorded that patent 1(b) had been completed for filing the same day.
309. Mr Ohlson was also asked about an email he sent on 22 October 2011 to Rodger Sargent (then a fellow director of TGL) addressing Dr Hill's stance on inventorship in which he said: "she doesn't really understand the patent process (...remember she didn't even read Kemp's drafting before submission)." Mr Ohlson suggested that he might have been speaking about ThermoLethal Vectors, but that was obviously not the case. It was suggested to Mr Ohlson that he could not have relied on anything Dr Hill said about the priority application if he knew she had not read it. However, as Mr Ohlson pointed out, she had indicated (including in her email of 29 January 2009) that she had read it and was happy with it. Further, Mr Ohlson knew, from receiving Dr Hill's letter of 11 August 2010, that she had read the PCT application and, while she had complaints about it, they did not include a claim of ownership. So even if Mr Ohlson had formed the view by October 2011 that Dr Hill had not read the priority application, that does not mean that TGL had not relied on Dr Hill's stated position.
310. In my judgment TGL did rely on Dr Hill's approval of the priority application when it filed it on 30 January 2009. In any event, Mr Ohlson's evidence was that TGL also relied on Dr Hill's position when "expend[ing] significant time and money developing the business into what it is today". It is plain that TGL did incur expenses (at least in terms of staff, premises and laboratory expenses, as well as patenting expenses) during the period until early 2012. I reject Dr Hill's submission that the onus was on TGL to prove that it would not have incurred such expenses had its rights been limited to thermophilic conditions. TGL would not have turned its mind to that question, as the suggestion that its rights were so limited did not arise until early 2012. In any event, it can be seen from the exchanges in 2010 (see paragraphs 290-293 above) and from Dr Porter's evidence that TGL was having trouble with thermophilic enzymes and in my view it can be inferred that it would not have taken the same course had it known that its rights were limited to thermophilic conditions.
311. TGL's expenditure continued after early 2012. However, by that point TGL was on notice of Dr Hill's claim. As to that, Mr Ohlson said that neither he nor anyone else on the board of TGL (or those of TIL and TDSL) regarded her claim as being serious, and her silence after 2012 was taken to mean that the claim had faded away. Whether the claim was taken seriously or not, or whether it was thought to have disappeared, I do not see how expenditure after early 2012 can have been committed in reliance on the assumption shared, or representations made, by Dr Hill before that date. After early 2012 TGL must have conducted its business knowing of Dr Hill's claim and taking the risk that it might be pursued and prove to be correct. That cannot be affected by the fact that TGL assessed the claim as not being a serious one.
312. On the other hand, I do not agree that TGL's continued expenditure after early 2012 once it became aware of Dr Hill's claim indicates that its expenditure before then was not in reliance on her previously stated position. Its continued expenditure can readily be explained by the fact that it did not take her claim seriously.
313. Mr Ohlson also said that, had the board of TGL believed that Dr Hill had an issue with the intellectual property position, it would have done whatever it sensibly could to remedy any issues including seeking a confirmatory assignment from Dr Hill. Ms McKechnie confirmed that by a confirmatory assignment Touchlight meant an assignment for no or nominal consideration. Kemps advised seeking such an assignment from the inventor(s) as a matter of course following filing of the priority application but it appears that no such assignment was sought from Dr Hill, at least until the national applications were being filed in late 2011. However, if Dr Hill had raised an issue about ownership of the inventions before early 2012, there is no basis for thinking that she would then have been prepared to execute such an assignment. Indeed, all the evidence suggests that she would have demanded significant value for an assignment if the issue of ownership had been live. In my judgment, the loss of the chance to seek a confirmatory assignment is theoretical rather than real.
314. As regards the position after early 2012, Mr Ohlson's evidence was that had Touchlight considered that Dr Hill's claim was serious, and there was any doubt about Touchlight's intellectual property position, Touchlight would have done whatever it sensibly could to remedy any issues including seeking a confirmatory assignment from Dr Hill. Quite apart from the points made in the previous paragraph, this evidence shows that Touchlight's failure to seek such an assignment at that time was not because of Dr Hill's stated position, but because of Touchlight's assessment of the merits of her claim.
315. As I said at the start of my consideration of the estoppel issue, there were differences between the parties as to the relevant law, and in particular whether the effect of any estoppel would be permanent or suspensory. If this matter should go further, and if the issue of estoppel (including the disputed points of law) becomes live, I believe that what I have said above is sufficient for the Court of Appeal to determine the estoppel issue and the form of any relief.
316. Touchlight submitted that Dr Hill's claim in respect of the three EP(UK)s was barred by s.37(5) & (9) Patents Act 1977, which provide (so far as relevant):
"(5) On any such reference [i.e. one under s.37(1)] no order shall be made under this section transferring the patent to which the reference relates on the ground that the patent was granted to a person not so entitled...if the reference was made after the second anniversary of the date of the grant, unless it is shown that any person registered as a proprietor of the patent knew at the time of the grant or, as the case may be, of the transfer of the patent to him that he was not entitled to the patent.
(9) The court shall not in the exercise of any such declaratory jurisdiction [see s.37(8)] determine a question whether a patent was granted to a person not entitled to be granted the patent if the proceedings in which the jurisdiction is invoked were commenced after the second anniversary of the date of the grant of the patent, unless it is shown that any person registered as a proprietor of the patent knew at the time of the grant or, as the case may be, of the transfer of the patent to him that he was not entitled to the patent."
317. Dr Hill submitted that these provisions only prevented an order for transfer being made and did not prevent the court making the orders which she sought, namely that she be registered as one of the proprietors of the EP(UK)s and that a retrospective exclusive licence to her be granted in respect of processes operated at mesophilic temperatures.
318. Touchlight responded by pointing out that s.37(5) is one of the provisions which are stated by s.130(7) to be so framed as to have, as nearly as practicable, the same effect in the UK as the corresponding provisions of certain international conventions, and to the observation of Jacob LJ in Yeda R&D Co Ltd v Rhône-Poulenc Rorer Intl Holdings [2006] EWCA Civ 1094 that s.37(5) was intended by Parliament to have the same meaning as the corresponding provision of the CPC.
319. Art. 23 CPC provides:
"1. If a Community patent has been granted to a person who is not entitled to it under Article 60(1) European Patent Convention, the person entitled to it under that provision may, without prejudice to any other remedy which may be open to him, claim to have the patent transferred to him.
2. Where a person is entitled to only part of the Community patent, that person may, in accordance with paragraph 1, claim to be made a joint proprietor.
3. Legal proceedings in respect of the rights specified in paragraphs 1 and 2 may be instituted only within a period of not more than two years after the date on which the European Patent Bulletin mentions the grant of the European patent. This provision shall not apply if the proprietor of the patent knew, at the time when the patent was granted or transferred to him, that he was not entitled to the patent."
320. Touchlight submitted that Dr Hill's claim was to be made a joint proprietor of the EP(UK)s and that accordingly such a claim was barred (subject to the question of knowledge) both as a result of s.37(5), interpreted to have the same effect as Art. 23 CPC, and as a result of s.37(9).
321. The submissions on this issue were brief and I was not referred to any authority on the point (the point taken by Touchlight was not taken in the one case to which I was referred, namely Taylor v Lanarkshire Health Board BL O/556/21). Therefore, as it is not necessary to decide this point, I decline to do so, though I have considerable sympathy with Touchlight's submission.
322. However, in case this claim goes further, I should deal with the issue of knowledge. Dr Hill submitted that the evidential burden was on Touchlight to show that the persons to which the EP(UK)s were granted or transferred were, at the time, mistaken about Dr Hill's rights. I do not agree. Both s.37(5) and s.37(9) provide a limitation bar "unless it is shown that any person registered as a proprietor of the patent knew at the time of the grant or, as the case may be, of the transfer of the patent to him that he was not entitled to the patent". That clearly requires such knowledge to be shown to avoid the bar, and therefore the evidential burden must remain with Dr Hill.
323. Mr Ohlson's evidence was that his understanding had always been that Touchlight was entitled to the Patents, and that he believed that the other members of the boards of TGL and TIL shared his understanding, based on the fact that no member of those boards had ever suggested to the contrary and (as explained above) that no one took Dr Hill's claim seriously. Dr Hill suggested that Dr Shafir and Mr Lewis might have had different views, but that was based on what they are recorded as having said about inventorship, not ownership. Dr Hill has not come close to showing that TGL or TIL knew, at the relevant dates, that they were not entitled to the EP(UK)s.
324. Dr Hill's claim for unjust enrichment relates to monies received by TGL and/or TIL, in particular licence fees or royalty payments made pursuant to licence agreements entered into with third parties, which she says should have been received in whole or part by her as a joint proprietor of the Patents. It is said that such monies were paid under a mistake of fact and/or law, namely that TGL and/or TIL were the sole proprietors of the Patents and in a position to grant such licences without her consent.
325. Touchlight contended that TGL and TIL had not been enriched "at the expense of" Dr Hill. It submitted that it was necessary for there to be a "transfer of value" in the sense of a receipt of a benefit by the defendant from the claimant and a corresponding loss to the claimant through its provision of the benefit, citing Investment Trust Companies v HMRC [2018] AC 275 at [42]-[45]. Dr Hill, citing the same passage, submitted that the argument that, because licence fees and royalties were paid by third parties, TGL and TIL had not been enriched at her expense "takes an unjustifiably narrow view of restitution".
326. Dr Hill's opening skeleton argument referred me to what it called a conceptually similar claim in BSI Enterprises Ltd v Blue Mountain Music Ltd [2014] EWHC 1690 (Ch). There Richard Meade QC (as he then was) declined to decide the claim for unjust enrichment saying that (see [126]):
"...the Claimants' claim in restitution, if it exists at all, is right at the fringes of a developing part of the law and I do not think it would be right or useful to make a decision about the boundaries of such a claim on the basis of the somewhat incomplete argument I have received, in circumstances where I have already dismissed the Claimants' claim on conventional grounds."
327. I shall take the same course, given that I was not addressed orally on the point of law and the foundation for the claim of unjust enrichment does not arise given that my findings on the Timing Issue and the Contract Issue mean that Dr Hill is not entitled to be a joint proprietor of the Patents.
328. I should add that Touchlight also advanced a case of change of position. Mr Ohlson's unchallenged evidence was that the licence fees and royalties that Touchlight had received had been invested into the business to fund R&D and other business costs. Otherwise, neither party suggested that the facts relevant to this case differed from those relevant to the estoppel issue, and so it is not necessary for me to make any further findings of fact in order for this issue to be addressed on appeal if need be.
329. Touchlight submitted that if Dr Hill's claim for the relief sought were to succeed the effect of the court's order would be to confer on Dr Hill a benefit in the form of joint proprietorship of the Patents and an exclusive licence thereto, and damages or an account of profits, and to deprive Touchlight of the sums awarded to Dr Hill and the benefit of sole proprietorship while leaving it having incurred the expense of prosecuting the Patents and building up a business based on them. It submitted that the effect of such an order would be to give rise to two causes of action available to Touchlight, one for breach of director's duties and one for unjust enrichment, that such claims would be of equal value to Dr Hill's claims, and that Touchlight had a defence of circuity of actions (citing Aktieselskabet Ocean v B Harding & Sons Ltd [1928] 2 KB 371 at 385).
330. Dr Hill submitted that both claims relied on by Touchlight were misconceived, for reasons that were also relied on in relation to the estoppel issue. Neither party addressed me orally on the law or the facts relating to circuity of actions, and neither party added to their opening skeleton argument in their written closing submissions. Nor did either party suggest that I needed to make any findings of fact over and above those relevant to the estoppel issue to enable the case of circuity of actions to be addressed on appeal if that becomes necessary. In those circumstances, and because I have held that Dr Hill's claim fails both on the Timing Issue and the Contract Issue, I do not propose to lengthen this judgment by addressing circuity of actions.
331. For the reasons explained above, Dr Hill's claim fails and must be dismissed and so Touchlight's counterclaim does not arise.
332. When I sent my draft judgment to the parties I asked them to provide me with proposals for redactions to be made to the judgment in the light of TGL's claim to privilege, together with written submissions in support of those proposed redactions. I received proposed redactions from Touchlight together with written submissions in support. Touchlight informed me that Dr Hill had no objections to its proposed redactions. The redactions proposed by Touchlight are shown by highlighting in the Annex to this judgment.
333. Touchlight submitted that I should apply the approach set out by Birss J in Unwired Planet Intl Ltd v Huawei Technologies Ltd [2017] EWHC 3083 (Pat) at [23]-[24], following a review of the authorities:
"23. Unless the public can see and understand a judge's reasons they cannot hold the courts to account. There is therefore a strong principle that all parts of a judgment should normally be publicly available. Nevertheless there are occasions on which judgments may be redacted. Redactions will require powerful reasons, supported by cogent evidence which addresses the details. Generalities will not do. Although redactions will be rare indeed when looking across the legal system in general, certain kinds of proceedings may regularly involve redactions due to the nature of the proceedings and the material involved. In any event however redactions must be kept to the bare minimum.
24. Factors which will be relevant include:
i) the nature of the information itself: for example cases in which some redaction may more readily be accepted could include technical trade secrets and private information about family life.
ii) the effect of the publication of the information. This will be a critical factor. If publication would be truly against the public interest then no doubt the information should be redacted. If publication would destroy the subject matter of the proceedings - such as a technical trade secret - then redaction may be justified. The effect on competition and competitiveness could be a factor but will need to examined critically.
iii) the nature of the proceedings: for example privacy injunctions and competition law claims may require some redaction while an intellectual property damages claim may not. The point is not that different kinds of case demand a different approach, it is that the balance of factors will change in different cases (e.g. the need to encourage leniency applications in competition law).
iv) the relationship between the information in issue and the judgment (as well as the proceedings as a whole). Obviously judges do not deliberately insert irrelevant information into judgments but not every word of a judgment is as important as every other word. It may be that some sensitive information can be redacted without seriously undermining the public's understanding of the reasons.
v) the relationship between the person seeking to restrain publication of the information and the proceedings themselves (including the judgment). For example, a patentee seeking damages for patent infringement on a lost profit basis knows that they will have to disclose their profit margin in the proceedings and that those proceedings are public. A third party whose only relationship with the case is that they are a party to a contract disclosed by one of the parties to the litigation is in a different position."
334. Touchlight also referred me to the judgment of the Court of Appeal in JC Bamford Excavators Ltd v Manitou UK Ltd [2023] EWCA Civ 840 regarding the impact of the Trade Secrets Directive on the exercise of balancing a party's private interest in protecting its confidential information and the public interest in open justice. However, Touchlight did not submit that the material which it proposed for redaction contained trade secrets, nor that I should apply an approach different from that set out in Unwired Planet v Huawei, whether because of JC Bamford v Manitou or because the material in question in this case was privileged.
335. Touchlight took the decision to disclose TGL's communications with Kemps in these proceedings, listing the documents in its disclosure lists and saying that they were privileged but Dr Hill was entitled to inspect them. Touchlight also relied on communications between TGL and Kemps, and the work done by Kemps for TGL, in support of its case. Touchlight called evidence from Dr Nicholls, as well as Dr Porter, which addressed, inter alia, Document A, the 5 June 2008 email exchange, the September 2008 draft application, the 27 October 2008 meeting and the 3 November 2008 letter, the November 2008 calls and the priority application as well as matters surrounding those documents and events. Document A and the 5 June 2008 email exchange were also addressed by Prof. Wittig in his reports.
336. In those circumstances it appears clear to me that Touchlight waived privilege in the communications which it disclosed, for the purpose of these proceedings. Further, it took the decision to deploy otherwise privileged material in its defence of these proceedings. It says that it was essential for it to do so. I accept that if it had not done so, that may well have hindered its defence, but that was still its decision. If documents which would otherwise be privileged form part of the reasoning in my judgment then I do not see how, having waived privilege for the purpose of the proceedings, Touchlight could pray in aid their privileged status to support their redaction from the public version of the judgment.
337. However, my understanding of Touchlight's submissions are that it does not seek to do that, as such. Instead, it asks me to treat the documents as confidential materials and apply the approach of Birss J in Unwired Planet v Huawei.
338. Touchlight submitted that it had sought to keep the level of proposed redactions to the minimum and had been mindful of the balance to be struck between the risk to TGL of loss of privilege by publication and the public's ability to understand the reasons for my decision. However, I have no doubt that if redactions at the level proposed by Touchlight were made to my judgment, that would seriously impair the ability of the public to see and understand the reasons for my decision. Indeed, substantial and significant parts of my judgment would be rendered incomprehensible.
339. Birss J explained in Unwired Planet v Huawei the need for "powerful reasons, supported by cogent evidence which addresses the details" to justify redactions from a judgment. Touchlight's submission was that, in any future proceedings in which the validity of its patent rights were challenged, it would be unable to claim privilege in any material included in my judgment. That, it submitted, could prejudice it if the material impacted on questions of validity. However, it did not seek to explain how each of the redactions which it sought was of material which could impact on questions of validity or would otherwise damage Touchlight if published.
340. Nevertheless, when considering the redactions sought by Touchlight, I have had in mind whether the material in question could reasonably be thought to impact on questions of validity or otherwise be liable to damage Touchlight if published. In my view it is also right to distinguish between communications between TGL and Kemps which relate to matters of technical fact, especially matters which appear in other documents referred to in this judgment, and communications which involve Kemps giving advice as to patentability or freedom to operate. I have of course also had in mind the degree to which the material is important to the arguments and my reasoning, which will affect the extent to which the public is able to understand my judgment notwithstanding any redactions.
341. I have set out my decisions on the redactions sought, and the reasons for those decisions, in the Annex to this judgment.
342. As will be seen, I have accepted only a small proportion of the redactions proposed by Touchlight. I appreciate that Touchlight may seek to appeal against my decision on redactions. Therefore, pending any appeal it will be necessary for the version of this judgment which is released to the public to contain all the redactions that Touchlight wishes to pursue on appeal. It is in the public interest that any appeal against my decision on redactions be lodged as soon as possible, to reduce the period of time during which the public will only have access to a version of my judgment which contains what are, in my view, excessive redactions. In respect of my decision on redactions I therefore will not take the normal course of directing that time for appealing shall not run until the hearing on the form of order to be made consequential on my judgment.
343. In relation to all other aspects of my judgment, I direct that time for appealing shall not run until the hearing on the form of order. At that hearing it will be necessary, inter alia, to address the proper scope of redactions that should be made to the public versions of the statements of case, witness statements, expert reports, transcripts and written submissions. Further, the parties should consider whether it would be appropriate to include in the order a general liberty to apply to allow any member of the public who regards my judgment (even on my view as to the proper level of redactions) to be over-redacted to challenge that - see Optis Cellular Technology LLC v Apple Retail UK Ltd [2024] EWHC 197 (Ch) at [56]-[57].
344. After the draft of my judgment on redactions was circulated to the parties, Touchlight informed me that it would not seek to appeal against my decision on redactions. Therefore, the public version of this judgment will be redacted in accordance with my decision on redactions.
ANNEX
|
Touchlight's proposed redactions |
Decision and reasons |
|
THE TIMING ISSUE
May - mid October 2008
The 9 May 2008 meeting with Kemps and Document A
110. The first substantive meeting between TGL and Kemps took place on 9 May 2008. There is no note of that meeting in disclosure, though Mr Ohlson said in an email on 12 May that TGL had briefed Kemps in great detail on every aspect of its technology including the future research direction in order to secure patents. |
Rejected. I do not see how this could have been privileged - the email of 12 May was to a potential investor in TGL. In any event the proposed redaction is of material the publication of which cannot realistically be said to be likely to damage Touchlight. |
|
111. [The entire paragraph]
112. [The entire paragraph]
113. There was an argument about the language used in this passage. Dr Hill submitted (with echoes of her submission on the Investor Presentation) that "insert telomeric ends into the linear DNA vaccine product" meant that the protelomerase must be acting directly on the concatemers. Touchlight responded by saying that the "DNA vaccine product" must be the material intended for administration to patients. In my view that all involved too detailed an analysis of language used in a document which was not a draft patent application but a document intended to enable the search agents to identify relevant prior art. |
Rejected. As can be seen from the unredacted parts of paragraph 113, these paragraphs concern a document sent to the search agents (i.e. Document A). The first two sentences of paragraph 111 merely explain what Document A was. The remainder of that paragraph, and paragraph 112, outline TGL's proposed technology in a manner which is no more detailed than appears from the discussion of various other documents in this judgment. The publication of that material (and the references to it in paragraph 113) cannot realistically be said to be likely to damage Touchlight. |
|
114. Dr Hill also relied on the skeleton claims at the end of Document A. Claim 1 was:
"An in vitro high temperature cell-free process for production of a DNA vaccine comprising:
a) contacting a DNA template with one or more primers and a thermophilic DNA polymerase;
b) incubating the DNA template under conditions promoting DNA replication by displacement of replicated strands through strand displacement replication of another strand,
wherein the DNA template comprises a sequence of interest but is devoid of bacterial plasmid replication sequences and/or CpG motifs."
Claim 3 was:
"The process of claim 1 or 2 further comprising:
(c) incubating the DNA vaccine product of b) with a thermophilic protelomerase to insert telomeric ends into the DNA."
115. Dr Hill submitted that the product of step b) would be a concatemer, with which the protelomerase is incubated in step c) to produce dbDNA. Hence, Dr Hill submitted, the skeleton claims are to the Close-Ended Process. Again Touchlight responded by noting that the starting point of step c) is a DNA vaccine product, which it said was something capable of being a DNA vaccine rather than a concatemer.
116. Again, this all seemed to involve subjecting the skeleton claims to a level of analysis which was not justified. As Dr Nicholls explained, the purpose of preparing the skeleton claims was to use words that might end up being used in claims to enable the search agents to identify keywords and classification codes, and to draft them broadly to catch as much prior art as possible. |
Rejected. The submission that the skeleton claims in Document A were to the Close-Ended Process was a significant one and it is important for the public to be able to understand the basis for that submission and the reasons given for rejecting it. In my view it is not realistic to say that publication of the skeleton claims (which were compiled for the reasons explained at the end of paragraph 116) and the submissions based on them is likely to damage Touchlight. |
|
117. Of course, Document A could only reflect the Close-Ended Process if that process had not only been conceived by May 2008 but also communicated to Kemps. Dr Hill said in her first statement that, at the first substantive meeting with Kemps, she explained that the process would be a two-step thermophilic one, involving a thermophilic polymerase for the amplification step and a thermophilic protelomerase for the step of insertion of the telomeric ends. However, Dr Nicholls said that he did not recall and did not believe that such a two-step process was discussed at the initial meeting, but instead developed as the patent applications were drafted (see further below). In his oral evidence he was clear about this. It was put to him that in her 5 June 2008 email (see below) Dr Hill was saying that the protelomerase could be used directly on the concatemers. His response was:
"That concept was never conveyed to us at any meeting with Dr Hill or in any of the project materials that I use for drafting."
118. In her second statement Dr Hill said that she believed that "we settled on the skeleton claims...through a mixture of emails and meetings" and in her oral evidence she claimed to have been "heavily involved" in the creation of the skeleton claims, including in phone calls with Kemps. However, there was no record of any such calls, Dr Nicholls did not recall any discussion with TGL about how the skeleton claims should be drafted, and it is apparent from the documents that Kemps sent Document A to the search agents without reference to TGL.
119. In her first statement Dr Hill said that at the 9 May 2008 meeting (or a subsequent meeting, though there is no further meeting that would fit into the chronology) she spoke to Dr Ali while Dr Porter was speaking to Mr Woods about something else (and Dr Nicholls was not present). She said, inter alia:
"I remember drawing out the 2-step process for him on a piece of paper and showing him how the restriction enzyme site that is used in the Open-Ended Process is replaced with a protelomerase site in the Close-Ended Process and how the action of the protelomerase on the concatemers results from the amplification step cuts the concatemers and closes the ends of the single units to form a closed "doggybone" shaped linear structure."
She adhered to this story in her oral evidence, embellishing it with descriptions of how she remembered the light coming through the window. She also said that Dr Ali had made notes of their discussions on his computer. However, as Dr Hill recognised, there was no file note of that discussion in disclosure. |
Rejected. The question of whether the Close-Ended Process had been communicated to Kemps prior to November 2008 was an important one on the Timing Issue. In order to understand my reasoning for rejecting that, it is important for the public to be able to see the key evidence and my assessment of it. Further, my findings are to the effect that the communications alleged to have taken place between TGL (in the form of Dr Hill) and Kemps referred to in these paragraphs did not in fact take place and so I do not understand how privilege could have attached to them. In addition, the technical information which is sought to be redacted is to be found elsewhere in this judgment. |
|
The 5 June 2008 emails
122. On 5 June 2008, while waiting for the results of the landscape searches, Mr Woods emailed Dr Porter, Mr Ohlson and Dr Hill. The email finished:
"Finally, we have a question for Vanessa before our meeting. In the DNA vaccine process technology project, there is mention of use of protelomerase. This is used to convert linear DNA vaccine molecules into closed structures which will not concatamerise/provoke a non-specific immune response. Would the protelomerase enzyme be present during the step of DNA replication or alternatively, would it be added in a final step to convert all vaccine molecules into closed structures?"
123. Dr Hill responded 90 minutes later, saying:
"It is most likely that we will add a thermophilic version of protelomerase as a final step. We need to let the replication stage go unhindered first, then treat with the protelomerase. We may be able to include both the DNA polymerase and the ProTL together in the reaction if the required reaction temperatures allow us to do one step followed bya second step using a different temperature of incubation."
124. Dr Hill's submissions relied heavily on her reply to Mr Woods' question. It was submitted that the language used to describe the first option, "let the replication stage go unhindered first, then treat with the protelomerase", suggested that there was nothing between those two steps. Further, it was suggested that the language used to describe the second, one pot, option indicated that there were only two steps, because only two enzymes, steps and temperatures were mentioned.
125. The first point to make is that (contrary to what Dr Hill said), Dr Hill's email does not show that she had the Close-Ended Process in mind. Her submission relies once again on parsing language which cannot have been chosen with the point now under debate in mind - the question she was asked was about a different issue (would the protelomerase be present from the outset or only at the end). Prof. Wittig said that, if he had not read other documents, he could see how reading what Dr Hill said about the first option might suggest direct action of protelomerase on the concatemers. But my task is not to consider this document on its own but to assess it together with the remainder of the evidence.
126. Prof. Wittig was scathing about the one pot suggestion - regardless of whether the two-step Close-Ended Process was envisaged or the Cut and Ligate Process. His view was that including the protelomerase in the reaction mixture during RCA would hinder amplification because it would bind to the template. Of course, my task is to assess what Dr Hill meant, not whether her proposal was technically sound, but Prof. Wittig's evidence does suggest that her answer was not fully thought through. |
Rejected. The 5 June 2008 emails were a major part of the case on the Timing Issue. I do not see how the public can begin to understand the argument, let alone my reasoning, without being able to see what the emails said. Further, the question and answer in the 5 June 2008 emails (and the arguments based on them) relate to purely technical matters and the language used to convey them, rather than advice by Kemps. I do not see how it can realistically be said that publication of the material proposed to be redacted could damage Touchlight. |
|
Further meetings with Kemps
128. The results of the landscape searches, which were broadly positive, were discussed at a further meeting on 13 June 2008. There is a note of the meeting prepared by Kemps, but neither party said that its contents advanced their case on the Timing Issue.
129. A further meeting with Kemps was held on 14 July 2008. Kemps' note of the meeting does not record who was present, and Dr Hill said that she did not recall it, but the documents show that it was rearranged specifically so that she could attend, so it is likely that she did. The note records a number of points under the heading "DNA vaccine process technology: Process patent" including:
"a) Number of essential method steps; order in which they can be performed.
b) Reaction components - enzymes/other proteins; all publicly available? If not, how can be obtained e.g thermophilic protelomerase. Reaction conditions - basic protocol / requirements for each element to work
c) Structure of template; basic elements of expression construct.
d) Structure of initial RCA product; downstream processing to give final product."
It therefore appears that all these matters were discussed at this meeting. If Dr Hill had the Close-Ended Process in mind, it is inconceivable that it would not have been discussed.
130. Following that meeting, Mr Woods indicated that Dr Nicholls would start work on drafting the patent applications and would send them out for review by Dr Hill and Dr Porter at the start of August (in fact, as will appear, the drafts were not circulated until early September, after Dr Nicholls returned from holiday). |
Rejected. I do not see how publication of the first part of paragraph 128, or of the mechanics and timings addressed in paragraph 130, could damage Touchlight. The points set out in the note referred to in paragraph 129 provide the reader with context for the unredacted passage at the end of that paragraph. Further, they are all matters addressed in various other documents to which I refer in this judgment. |
|
The September 2008 draft patent application
145. On 5 September 2008 Dr Nicholls sent Dr Porter, Mr Ohlson and Dr Hill a draft of the DNA vaccine process patent application which he described as "focussing on the various concepts that are encompassed in [TGL's] process technology". He explained that he had included the doggybone (closed linear DNA) concept and had added claims to doggybone expression constructs as DNA molecules per se, and to their use in therapy. He then set out various questions regarding technical aspects of the process, including whether thermophilic restriction enzymes would be of particular use after the RCA step. He concluded by saying that he looked forward to comments and answers on his questions, together with input in describing the invention in a greater level of detail.
146. Of relevance to the Timing Issue is that, on pages 5-6 of the draft application, the following passage appeared:
[The entire quoted passage]
147. Then, on pages 8-9, the following passages appeared:
[The entire quoted passage]
148. [The entire paragraph]
149. As will be seen, the draft patent application clearly disclosed and claimed the Cut and Ligate Process and neither disclosed nor claimed the Close-Ended Process. That is consistent with Dr Nicholls' evidence that Kemps had not been told about the Close-Ended Process by this stage.
150. Dr Hill's case is that Kemps had been told about the Close-Ended Process in May 2008 - that is the basis for her contention that the Close-Ended Process can be seen in the skeleton claims in Document A. So her case has to be that Dr Nicholls had forgotten about the Close-Ended Process by the time he came to write the draft patent application, and had replaced it with the Cut and Ligate Process (she submitted that it was Dr Nicholls who had come up with that idea). That is inherently unlikely. Dr Hill pointed out that Dr Nicholls was still training as a patent attorney and that, at the time the draft patent application was circulated, Mr Woods was on holiday (it was at that point that Dr Ali was copied in on emails). However, Dr Nicholls' evidence was that he drafted the application with guidance from Mr Woods and that he started with the claims, on which Mr Woods was "fairly pernickety". So for the patent application to have ended up in the form it did would require not only Dr Nicholls, but also Mr Woods, to have forgotten about the Close-Ended Process, which is even less likely.
151. In support of the suggestion that Dr Nicholls had somehow forgotten the Close-Ended Process and come up with the Cut and Ligate Process, Dr Hill pointed out that the draft patent application does not contain the one pot possibility which was mentioned in her email of 5 June 2008 (a possibility which does appear in the PCT application). However, Dr Nicholls said that his recollection was that at the time they had understood from the email that the protelomerase was most likely to be used as a final step. I cannot see how the omission of the one pot option from the September 2008 draft is an indication that Dr Nicholls had forgotten the Close-Ended Process and come up with the Cut and Ligate Process. |
Rejected. The September 2008 draft application is (along with the August 2008 slide deck) a key plank of Touchlight's case that before the Service Agreement came into effect, Dr Hill had not conceived of the Close-Ended Process but instead the Cut and Ligate Process. Redaction of the relevant contents of the September 2008 draft application, whether the text itself or the summary of it, would make it hard for the public to understand the argument and my reasoning.
Further, the contents of the September 2008 draft application that Touchlight seeks to redact does not appear to contain any material that is not in substance apparent from various other documents referred to in this judgment. In addition, the subject-matter of the September 2008 draft application was either abandoned by Touchlight or carried forward into the priority application. I cannot see how publication of the passages sought to be redacted could realistically be said to damage Touchlight.
In so far as paragraph 151 refers to the contents of the 5 June 2008 emails, my comments above apply. |
|
Late October 2008 - January 2009
The 27 October 2008 meeting
157. Kemps' hand-written notes of the meeting of 27 October were disclosed. They include: "Substrate for ProT needs to be covalently closed i.e. circular, Vanessa to confirm this". Similarly, Kemps' letter of 3 November 2008 summarising the matters discussed at the 27 October meeting says: "Please can you also confirm whether it is appropriate for claim 9 to be dependent on claim 3, or if in practice ProT only works on religated circular templates (claim 5)."
158. It therefore appears that at the meeting of 27 October a question arose as to whether protelomerase required a circular substrate, and that the outcome of the discussion was that it did, but Dr Hill would confirm. I agree with Touchlight that if Dr Hill had been aware that protelomerase also operated on linear DNA and so did not need a circular substrate, it is surprising that she did not mention that at the meeting when the question was posed.
161. However, even if Dr Hill had not read the draft patent application in advance of the meeting, it is hard to see why she would have been baffled and confused by the question of whether protelomerase needed a circular DNA substrate. That would have been an easy technical question for Dr Hill to answer (if she knew the answer) regardless of whether she had read the draft patent application and regardless of whether she knew the reason for the question being asked. |
Rejected. The question raised at the 27 October 2008 meeting, and Dr Hill's recorded response to it, were a key part of Touchlight's case on the Timing Issue. Redaction of the question and the response would make it hard for the public to understand the argument and my reasoning. Further the material in question concerns technical fact rather than any advice given by Kemps. I cannot see how publication of the passages sought to be redacted could realistically be said to damage Touchlight. |
|
The 5 November 2008 call and the 7 November 2008 email
163. On 4 November 2008 Dr Porter emailed Dr Nicholls to thank him for the meeting summary, saying that they would start to put together the additional information that he had requested. He also emailed Dr Hill to confirm that they were due to meet on 10 November to prepare a response to Kemps and to ask Dr Hill if she could "come up with some "doggybone" designs based on some of your thoughts at the Kemp meeting".
164. On 5 November 2008 Dr Porter called Dr Nicholls. Dr Nicholls' file note of that conversation starts as follows:
"Neil telephoned to discuss the "improved expression cassette" concept. He indicated that following his review of the mechanism of action of protelomerase (ProT), Touchlight would want to cover an additional aspect in the proposed application. ProT has a combined endonuclease and ligase activity, such that it can cleave and rejoin double stranded DNA molecules without the need for separate use of restriction enzymes and DNA ligases. Thus, we should add a claim to a process for making the expression cassette molecule which involves the direct generation of the expression cassettes from material amplified by DNA polymerase. In particular, where the DNA application is rolling circle, the concatamers that are generated can be directly resolved into the expression cassette molecules using protelomerase and no other resolving enzymes."
165. As will be apparent, what Dr Nicholls records Dr Porter as having described on this call was the Close-Ended Process. Dr Nicholls' evidence was that this was the first time that the concept of having the protelomerase act directly on the amplified material was raised, and that he recalled thinking that [xxxxxxx xxxxxx xxxxx xxxxx xxxxxx xxxxx xxxxxxx xxxxxx xxxxxx xxxxxxxxx]. His recollection of his reaction about [xxxxx xxxxx xxxxx xxxxx] is inherently plausible and is consistent with his recommendation, also recorded in his file note, to separate off protelomerase aspects to a separate application.
166. Dr Porter had no real recollection of the events surrounding this call. Dr Hill's evidence fluctuated somewhat, but the thrust of it was that shortly before 5 November she and Dr Porter had sat down at the end of the lab to run through the draft patent application (she said that was the first time she had read it) and she was shocked to notice that the document contained material based on her draft RHUL papers and mistakenly referred to ligation of the single units before the protelomerase step. She said she told Dr Porter that needed to be corrected, and that they had a further conversation by telephone about it on 5 November, at which she reiterated the mistake and the need for correction. She said that Dr Porter was embarrassed, tense and angry but promised to call Kemps to explain the position.
167. On Friday 7 November 2008 Dr Hill emailed Dr Porter as follows:
"After our conversation Wednesday, I realised that we had gone a bit off track with the RCA patent. I suspect this is due to such a long break since we last saw Jamie [sic] and being so thinly spread with so much other stuff to do at this time. I think what he has done, is concentrated on the RCA papers I wrote and has not looked at the technical plan we gave him. The papers really relate mostly, to how to clone something into our linear cassette and the technical plan relates to the actual vaccine production process. The original technical plan (attached) from Dec '07 clearly relates that the Protelomerase is used to cut and ligate the ends directly after the RCA amplification step and hence we did not propose to use restriction enzymes or a ligase. In the light of this and the new idea of splitting this patent into 2, I think we need to sit down together with the 3 separate areas of work (the 3rd being the cassette design) and work out how best to organise these 1/2 patents. Once we have sorted this one then we can move to the TLVector system. Hope this all makes sense!"
172. Dr Hill submitted that it was critical to Touchlight's case that it was Dr Porter that conceived of the Close-Ended Process in early November 2008. It was said that there were only two options - Dr Porter conceived the invention then and called Kemps (either before or after speaking to Dr Hill about it) or Dr Porter discussed matters with Dr Hill and called Kemps having appreciated that the draft patent application had failed to reflect the Close-Ended Process which had been conceived of by Dr Hill long before then. I do not agree that those are the only two options or that Touchlight's case relies on the first option being true. Touchlight's case is that the Close-Ended Process was conceived of by Dr Hill in early November 2008. That is not inconsistent with Dr Porter then calling Kemps to tell them about it following a discussion with Dr Hill. |
Rejected, save as indicated below in relation to paragraph 165.
The 5 November 2008 call and the 7 November 2008 email were at the core of the case on the Timing Issue. Unless their contents are included in the judgment the public will not be able to understand my reasoning.
Paragraph 163 does not contain any material the publication of which could damage Touchlight.
As to paragraph 164, the 5 November 2008 file note does not record any technical matter or communication between TGL and Kemps that is not apparent from the priority application.
As to paragraph 165, Dr Nicholls' evidence as what was mentioned on this call for the first time was important on the Timing Issue. I do not accept that the material about mechanics at the end of paragraph 165 could be damaging to Touchlight. However, I am prepared to redact the words after "thinking that" in the second sentence, and those between "reaction about" and "is inherently" in the third sentence of paragraph 165. I do not believe that doing so will materially hinder understanding of my judgment.
As to paragraph 166, see my comments above relating to the September 2008 draft application.
As to paragraph 167, the passages sought to be redacted compare the September 2008 draft application (as to which see above) to documents which are not said to be confidential and are addressed elsewhere in my judgment or relate to mechanics. I note that no privilege was claimed in the 7 November 2008 email in any event.
The decision on paragraph 172 flows from the above. |
|
The 12 November 2008 call
173. On 12 November 2008 Dr Porter called Dr Nicholls. Dr Nicholls' manuscript notes of the conversation were disclosed, as was his file note. The file note records that Dr Porter had called to update him following a TGL meeting on 10 November and goes on to say:
"In regards to the second patent application (improved expression cassette; our ref: N.106698), he indicated that Touchlight were particularly excited about this project. The process Touchlight have in mind is using a closed linear DNA molecule as a starting template, which would then be denatured and amplified, preferably by RCA. The denaturation step converts the closed linear molecule into a circular molecule, and so RCA can be used to amplify these circle forming long concatamers.
The key point is that ProT can be used to resolve such concatamers directly without need for additional enzymes, as discussed in our 5 November 2008 phone call. [xxx xxx xxx xxx xx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxx xxxxx xxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxx xxxxx xxxx xxx xxxx]"
174. The manuscript notes are consistent with the file note. They include "Linear DNA with telomeric ends as starting material ... Heat -> converts doggybone -> circular molecule in cassette, have Pro T binding sites ... [then do RCA via mech of 1st patent - long concatamers of original material] ... Pro T resolves directly (no need for extra enzymes)".
175. This is the first record of the idea of using doggybone DNA as a template for RCA and accordingly of the dbDNA Template Process. Dr Nicholls' evidence was that this was, as far as he could recall, the first he had heard of this concept, and that is consistent with his contemporaneous notes. |
Rejected, save to the extent indicated below in relation to paragraph 173.
The 12 November 2008 call was also important to Touchlight's case on the Timing Issue. Unless the public are able to see the key aspects of the notes and Dr Nicholls' evidence they will not be able to understand my reasoning. Further, the technical matters referred to in the notes are to be found in the priority application. However, some of the quote in paragraph 173 consists of advice which it is not necessary to see in order to understand my reasoning. I will therefore redact the second paragraph of the quote after "phone call." |
|
The November 2008 slide deck
177. This slide, like the notes of the conversation between Dr Porter and Dr Nicholls on 12 November, contains the idea of using doggybone DNA as starting material for RCA.
180. Plainly this slide deck is supportive of Touchlight's case. The differences between the comparable slides in the August 2008 and November 2008 slide deck strongly suggest a change in the proposed process, from one in which the concatamers were cut with restriction enzymes before the ends of the single units were closed using protelomerase to one in which protelomerase was used directly on the concatamers to cut and close the ends. Further, it is consistent (as are Dr Nicholls' file notes and evidence) with the Close-Ended Process and the dbDNA Template Process having been conceived in early November 2008. Dr Hill's attempts to explain the changes between the August 2008 and November 2008 slide decks were not at all credible. |
Rejected, for reasons given above in relation to the 12 November 2008 call. |
|
The inventorship dispute
185. The issue appears to have been prompted by Mr Woods sending Mr Ohlson a letter on 19 February 2009 noting that the priority application had been filed without a statement of inventorship and asking him to consider whether anyone else, apart from Dr Hill, qualified as an inventor (providing Kemps' circular on inventorship by way of guidance). He also recommended that the inventor(s) should execute a confirmatory assignment in favour of TGL and provided a suggested draft. |
Rejected. I cannot see how publication of the material in question (which provides context for what follows and is also in part relevant to the estoppel issue) could be damaging to Touchlight. |
|
190. On 27 March 2009 Mr Woods emailed Mr Ohlson to say that they would provide a note with their [xxxxxxx xxxxx xxxxx xxxxxx xxxxx xxxxxx xxxxx xxxx xxxx] in draft form early the next week. On 2 April 2009 Mr Woods sent the draft to Mr Ohlson, asking whether it met his requirements and offering to discuss any changes he would like. On 23 April 2009 Mr Woods chased Mr Ohlson for any comments. The finalised version (which appears to be identical to the draft) was sent to Mr Ohlson on 11 May 2009.
191. Kemps' letter of 11 May 2009 [xxx xxx xxx xxx xx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxx xxxxx xxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxxxx xxxxxxx xxxxx xxxxx xxxx xxxxx xxxxxx xxxxx xxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxxx]
192. Kemps' letter of 11 May 2009 [xxx xxx xxx xxx xx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxx xxxxxxxx]:
[The entire quoted passage]
193. [The entire paragraph]
194. On 20 May 2009 Mr Ohlson emailed Kemps' letter to Mr Lewis and Dr Shafir. Dr Lewis responded to say that he expected that Dr Hill would "challenge this to the bitter end". Mr Ohlson replied saying that he thought it was Dr Porter who "discovered [Heinrich] and put 2 + 2 together" (xxxx xxxxx xxxxx xxxxx xxxxx xxxxx) and took that core idea to Dr Hill. He concluded: "So the question we have to ask ourselves is if/when do we break it to Vanessa. As you rightly ask will she take it out on Neil or the company? My guess would be both (if she is still involved with the company then and if we have to disclose it to her)." |
Accepted. I bear in mind that the inventorship dispute was only said to be relevant by Dr Hill, and that it did not bear directly on the Timing Issue, but only really went to credit of Dr Porter. I also bear in mind that Kemps' letter of 11 May 2009 was a letter of advice, and I can see that publication of its contents has the potential to harm Touchlight if a dispute arises in which inventorship is a live issue. I do not believe that if the redactions proposed are made that will have a significant impact on the public's ability to understand my reasoning. |
|
201. In cross-examination, Dr Porter was asked about the contents of Kemps' letter of 11 May 2009. He said he was unable to remember what he had said to Kemps at the meeting on 26 March 2009. When asked about the part of Kemps' letter that addressed the [xxxxxxxxxx xxxxxxxxx xxxxx xxxxxx xxxx], he readily accepted that the idea of use of thermophilic conditions and proteins for the DNA amplification step had been Dr Hill's. When asked about what Kemps' letter said regarding [xxxxxxx xxxxxxx xxxxxxx], he did not seek to maintain that he had conceived of the Close-Ended Process, but he did maintain that he had conceived of the dbDNA Template Process (xxxxxxxxx xxxxxx xxxxxx xxxxxx xxxxx xxxxx xxxxx) - that, of course, is inconsistent with Touchlight's position in these proceedings.
202. Dr Hill submitted that Dr Porter must have provided a false account to Kemps on 26 March 2009. Touchlight submitted that he had merely been labouring under the misapprehension that identifying something as being patentable meant that he was an inventor. But Touchlight also accepted that Kemps would not have been labouring under that misapprehension. Further, it is apparent from Kemps' letter that they [xxxxx xxxxxx xxxxx xxxxxx xxxxxx xxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxx].
203. In my judgment the likelihood is that Dr Porter did indeed exaggerate his contribution when he spoke to Kemps on 26 March 2009 and claim to have conceived ideas that had in fact been conceived by Dr Hill. [xxxxxx xxxxx xxxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxxxxxxx] Further, the relationship between Dr Hill and Dr Porter was poor at that time, and it is entirely credible that Dr Porter would have wanted to claim credit for ideas that had come from Dr Hill.
204. Dr Hill suggested that if Dr Porter was wrongly claiming credit for inventions when he met Kemps on 26 March 2009, then one could also not trust what he said to Dr Nicholls on the calls in early November 2008. I agree that I should treat any claim by Dr Porter on those calls to have come up with the inventions with a degree of scepticism. But Dr Nicholls' notes do not record any such claim being made - the most one could say is that in the note of the 5 November call Dr Nicholls records Dr Porter as referring to "his" review of the mechanism of action of protelomerase having led to the idea. But the importance of the calls of 5 and 12 November lies not in the light they shed on who made the inventions, but on when the inventions were made. |
Accepted in part (bearing in mind the matters stated in relation to the previous group of paragraphs) but rejected to the extent, and for the reasons, that appear below.
As to paragraph 201, I do not see how publication of the second block of text can be damaging to Touchlight.
As to paragraph 203, in order to understand the aspect of my judgment that deals with the inventorship dispute, it is important that the public be able to see the whole of the first sentence. I do not see how publication of that sentence, at the level at which it is stated, could be damaging to Touchlight.
The same applies to the first sentence of paragraph 204. As to the remainder of paragraph 204, see my comments on the note of the 5 November 2008 call. |
|
206. Finally, some reliance was placed by Dr Hill on an affidavit that Dr Porter signed on 14 September 2018. That contained the following passage:
[The entire quoted passage]
207. The only aspect of this which was put to Dr Porter as being incorrect was the first sentence. It was suggested that there were in fact no such difficulties, but Dr Porter explained that he meant that no suitable thermophilic enzymes were available. The other point put to Dr Porter was that his affidavit did not say that the Close-Ended Process was his idea following a review of Heinrich, as he had said to Kemps in March 2009. But it does not assist Dr Hill to point out that in his 2018 affidavit Dr Porter did not make the exaggerated claim that he made to Kemps in March 2009 and not to take issue with the rest of the affidavit, which was consistent with Touchlight's case. |
Rejected. No privilege was claimed in Dr Porter's affidavit when it was disclosed by Touchlight. I cannot see how publication of the quoted parts of the affidavit (nor the relevant part of paragraph 207) in this judgment could possibly damage Touchlight. |
|
Overall assessment
210. My reasons are as follows: ...
(viii) The fact that the Close-Ended Process does not appear in the draft patent application circulated in early September 2008 is strong evidence that it had not been communicated to Kemps by that date. The suggestion that the Close-Ended Process can be deduced from Document A (and in particular the skeleton claims) relies on an over-linguistic analysis of a document which was not produced with the purpose of being subjected to such analysis. Dr Hill's email of 5 June 2008 provides (at best) only slight support for Dr Hill's case, when taken in isolation. Further, if the Close-Ended Process had been communicated to Kemps before Document A was produced in June 2008, that would require both Dr Nicholls and Mr Woods to have forgotten about it when drafting the patent application. That is inherently incredible.
(ix) No credible explanation has been advanced for why Dr Hill would have responded in the way she is recorded as having done in the meeting with Kemps on 27 October 2008 when asked whether the substrate for protelomerase needed to be circular, if she had conceived of the Close-Ended Process by that time. I do not accept her evidence that she was not aware of the contents of the draft patent application before that meeting; even if she had not been that would not explain her response.
(x) Dr Nicholls' notes of the calls with Dr Porter on 5 and 12 November 2008 are entirely consistent with his evidence that he had not been told about the Close-Ended Process or the dbDNA Template Process before those dates. |
Rejected. The reasons for doing so are apparent from what I have said above when considering the redactions relating to the September 2008 draft application, Document A, the 27 October 2008 meeting and the 5 and 12 November 2008 calls. |
|
THE CONTRACT ISSUE
The factual matrix
243. Dr Hill relied on the advice given by Kemps on 13 June 2008 after the receipt of the landscape searches. Kemps' notes of the meeting say this about patentability of the DNA vaccine process:
[The entire quoted passage]
244. While this advice does recognise that the [xxxxxx xxxxxx xxxxxx xxxxxxxxxxx xxxxx xxxxxx xxxxxx xxxxx x xxxxxxx xxxxx xxxxxxx xxxxxxx xxxxx xxxxxx xxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx] Further, it was preliminary advice rather than final definitive advice. I do not agree with Dr Hill's submission that as at the date of the Service Agreement it was the parties' objective expectation that the use of mesophilic conditions in the DNA vaccine project could not be patentable. In any event, the idea of using a protelomerase to produce a DNA vaccine (including, on the hypothesis on which I am working, using the Close-Ended Process and/or the dbDNA Template Process) would be confidential information belonging to Dr Hill (disclosed under the NDA, which gave TGL no right to use it). |
Accepted (save for the text before the quote in paragraph 243). While redaction of this material will to some extent impair the ability of the public to understand my reasoning, I bear in mind that the material in question is advice given by Kemps which could be damaging to Touchlight if it is published, and that it was Dr Hill who relied on the advice to try to establish an objective expectation. |
|
ESTOPPEL
Limb 1
Events leading up to the filing of the priority application
259. I have considered above whether Dr Hill was aware of the contents of the 5 September 2008 draft patent application before the meeting with Kemps on 27 October. I find that she was sufficiently aware of its contents to appreciate not only that it described the Cut and Ligate Process (and not the Close-Ended Process), but also that it described and claimed a process which was not limited to wholly thermophilic conditions. Certainly, given that Dr Hill had been asked to review the draft and had agreed to do so, TGL was entitled to assume that she had done so.
260. I have referred above to Kemps' handwritten notes of the meeting on 27 October 2008 and Kemps' letter of 3 November 2008 summarising the matters discussed at that meeting. The handwritten notes say this:
"(4) Temperatures
- Only thermophilic step is amplification step b)
- Ensure other steps can be non-thermophilic or thermophilic
- NB doesn't matter if temp is cooled after amplification as SSBPs prevent snap back of unmeshed concatamers - allude to this?"
Similarly the letter says this:
"3. Non-thermophilic steps
As explained in our meeting, claim 1 only requires a thermophilic amplification step. All other process steps can be carried out at any temperature. Also, the claim does not exclude the possibility of initial non-thermophilic and/or PCR-type steps. I will ensure that the description provides a disclosure that all steps other than amplification step b) may be thermophilic or non-thermophilic, and a list of appropriate temperatures."
261. It is also relevant to note that at this stage claim 14 was a product claim, to closed linear DNA having hairpin ends comprising telL and telR sites flanking a central double-stranded region. The handwritten notes record Dr Nicholls advising that [xxxxx xxxxx xxxxx xxxxx xxxxx xxxxx xxxx xxxxxxx xxx xxx xxx xxx xx xxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxx xxxxxxx xxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxxxx xxxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxxx xxxxxx xxxxxx xxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxxxx xxxxxx xxxxx xxxxx xxxxxx xxxxxxx xxxxxx] (original emphasis). Similarly, the letter advised that "claim 14 does not limit preparation of the product to a particular process; it covers the product made by any process. [xxxxxx xxxxxx xxxxxxxx xxxxxx xxxx xxxxx xxxxxx xxxxx xxxxxx xxxxx xxxxxxxx xxxxxxxx xxxxxx xxxxxxx xxxxx]"
262. It is clear from the handwritten notes that Dr Nicholls explained at the meeting on 27 October that the draft patent application was not limited to processes carried out wholly under thermophilic conditions. As mentioned above, Dr Hill said that she had not read the draft application before the meeting (which I have rejected) and that she was baffled and confused by some of the questions raised. The notes of the meeting indicate that she was engaged (and it appears from Dr Porter's email of 4 November 2008 that she came up with cassette designs at the meeting - see paragraph 163 above). Further, Dr Nicholls' explanation that the draft application was not limited to wholly thermophilic processes was not a question, let alone one which could have baffled and confused Dr Hill. She also suggested that she had been confused because Dr Porter had told her on the way to the meeting that there would be broad-ranging discussions and that nothing was set in stone, but at the meeting she found that everything had been set in stone. In fact, it is clear from the notes of the meeting that things were not set in stone. In any event, that does not grapple with the point, which is about whether she understood Dr Nicholls' explanations. I find that Dr Hill must have understood Dr Nicholls' explanations, but even if she did not, TGL was entitled to assume that she had.
263. In any event, Dr Hill was sent the letter of 3 November which reiterated those explanations. Dr Hill suggested that she had not read it, but it is inherently unlikely that she would not have read such a letter on a topic of great importance to TGL. Further, on 4 November Dr Porter emailed Dr Nicholls to thank him for the meeting summary, saying "I have spoken to Vanessa and Jonny and we agree with your recommendation to separate the product and process claims and submit two separate patent applications. We shall now start to put together the additional information you have requested. As you pointed out, we need to put some additional thought into inventive steps related to the products. We'll work on this and get back to you for opinion." This suggests that Dr Hill had considered the 3 November letter. I hold that she had. In any event, TGL was entitled to assume that she had done so.
264. In any event, it is clear that by 7 November 2008 Dr Hill had considered the draft patent application. She asserted that she had done so before then (though after the meeting of 27 October) and that is clear from her email of 7 November (on which she relies heavily in relation to the Timing Issue) - see paragraph 167 above. She must have appreciated, by then at the latest, that the draft application was not limited to processes operated under wholly thermophilic conditions. |
Rejected, save to the extent indicated below in relation to paragraphs 261 and 263.
I bear in mind that Touchlight's case of estoppel (which it said I should address in my judgment even if I had determined the Timing Issue and the Contract Issue in its favour) relied heavily on Dr Hill's approval of the filing of the priority application and her awareness of its contents and scope.
It would not be possible for the public to understand my judgment on this part of the case if the key aspect of the September 2008 draft application were to be redacted (as proposed in the second block of text in paragraph 259, the first and third blocks of text in paragraph 262 and in paragraph 264). Similarly, in my view the extracts from the notes and letter in paragraph 260 are necessary in order to understand paragraph 262, and I do not see how their publication could damage Touchlight.
As to the first block of text in paragraph 259 and the first block of text in paragraph 261, see my comments above relating to the September 2008 draft application. As to the second block of text in paragraph 262, see my comments on paragraph 163 above.
I am prepared to redact the second and third blocks of text in paragraph 261, save for the first sentence of the quote from the letter, because they contain advice given by Kemps and, if the first sentence of the quote from the letter is included in the judgment together with the first sentence of paragraph 261, it will be possible for the public to understand my reasoning.
I am prepared to redact the third sentence in the quote in paragraph 263 because it relates to the advice being redacted in paragraph 261, but not the remainder, which deals with mechanics. |
|
265. Following TGL's agreement to the proposal to submit two separate applications, on 27 November 2008 Dr Nicholls emailed Dr Porter a draft application relating to the improved DNA amplification aspects, together with a covering letter. The letter explained that all subject matter relating to the use of protelomerase had been removed and would be pursued in a separate application, and that the description contained basis for all steps other than the strand displacement step being carried out under non-thermophilic conditions (which indeed it did). Again Dr Hill suggested that she would not have had time to read the letter or the draft application and that she had not done so (at least properly). Again it is inherently unlikely that she would not have done so. Further, it is clear from an email and attachments sent by Dr Porter to Dr Nicholls on 3 December 2008, responding to the letter of 27 November, that she must have done so. For example, in one of the attachments Dr Porter says: "Vanessa has pointed out that, at present, our claims appear to cover only the use of a single DNA polymerase. ..."
266. On 12 December 2008 Dr Nicholls emailed Dr Porter, Dr Hill and Mr Ohlson a finalised version of the same application and a covering letter asking for comments and for agreement to place it on hold pending completion of the second application. Again Dr Hill suggested that she would not have read these documents. This time there is no documentary indication that she did what would have been expected of her, but in any event TGL was entitled to assume that she had.
267. On 18 December 2008 Dr Nicholls emailed Dr Porter, Dr Hill and Mr Ohlson to say that he had been reviewing material provided by Dr Porter (including cassette designs based on Dr Hill's November 2008 slide deck) and suggesting that, to assist drafting and save costs, Dr Porter and Dr Hill might prepare some descriptive text for the second application. Following discussion with Dr Hill, Dr Porter sent a draft to Dr Nicholls on 7 January 2009. Dr Nicholls then sent a draft application entitled "Production of Closed Linear DNA" to Dr Porter, Dr Hill and Mr Ohlson on 16 January 2009, together with a covering letter. The letter explained (original emphasis):
"As suggested, the application is now focussed on a process for production of closed linear DNA that involves use of DNA polymerase and protelomerase in combination. ...
The proposed claims are broadly directed to amplification based on any DNA template comprising a protelomerase target sequence, using any DNA polymerase, and a subsequent processing step for the amplified DNA using any protelomerase. Please let me have your comments on this proposed claim scope. ..."
That was an accurate summary of the accompanying draft application, which contained no temperature limitations and expressly contemplated the use of mesophilic polymerase and protelomerase. |
Rejected.
I repeat my opening comments on the previous group of paragraphs.
The text proposed for redaction in paragraphs 265 and 266 concerns mechanics and (at a high level) the contents of a draft application from 2008. I cannot see how its publication could damage Touchlight.
Paragraph 267 relates to a draft of what became the priority application (including mechanics for its preparation). The statements made about the draft apply equally to the priority application. I cannot see how publication of those statements could realistically be said to damage Touchlight. |
|
270. Notwithstanding this, Dr Hill said that she had not read Dr Nicholls' letter of 16 January 2009 nor the accompanying draft application (i.e. "patent 1(b)"). She said that she had no time, that she wanted the patents put on hold and that the decision at the meeting involved overriding her. I do not accept any of that. Further, Dr Nicholls' file note of a call with Dr Porter on 27 January 2009 indicates that Dr Hill had some concerns about [xxxxx xxxxxx xxxxx xxx xxxxx xxxx xxxx xxxxxx xxxxx xxxxx xxxxx xxxxxxx xxxxx xxxxx xxx], so it appears that Dr Hill had been engaging with the draft application.
271. On 28 January 2009 Dr Nicholls emailed Dr Porter, Dr Hill and Mr Ohlson a final draft of the application relating to production of closed linear DNA, asking for final comments on the specification or its approval for filing. As with the draft sent on 16 January, there were no limitations as to temperature.
274. Dr Porter passed on Dr Hill's request for the inclusion of "telomere resolvase" to Dr Nicholls on 29 January 2009. There was a further directors' meeting on 30 January 2009 at which it was recorded that tasks 1-3 (see paragraph 269 above) had been completed. The priority application was filed on the same day (as was the application relating to improved DNA amplification).
275. I find that Dr Hill was aware that the priority application contained no limitation as to temperatures at which the process for producing closed linear DNA could be operated, and indeed stated that it could be operated using mesophilic enzymes as well as thermophilic ones. Indeed, she had been aware since at least late October 2008 that the patent applications being drafted by Kemps were not limited to processes operated under wholly thermophilic conditions. Yet at no point did she raise any objection to TGL filing applications of such a nature in its own name. On the contrary, she approved the priority application being filed in TGL's name (at least on 29 January 2009 - Mr Ohlson said he recalled her telling him orally that she was happy with the draft on previous occasions as well). |
Rejected, save to the extent indicated in relation to paragraph 270.
I will allow redaction of the text between "concerns about" and "so it appears" in paragraph 270. I can see that publication of the intervening material could possibly damage Touchlight, and it is not necessary for the public to see the details to understand my judgment.
The text proposed for redaction in paragraphs 271, 274 and 275 either relates to mechanics or is material which is apparent from the priority application. Further, my comments on paragraphs 259-264 relating to the key relevant feature of the September 2008 draft application apply equally here. |
|
Dr Hill's concerns about the direction of TGL following her resignation
288. On 3 July 2009 Dr Hill sent a letter to the board in which she said:
"As I am a Director and the major share holder of [TGL], it is obviously in my interests that the company does well. It is also obvious that I should want to that see that my original, lifetimes work is being furthered in the best manner possible for this company's interests."
She went on to express concern that insufficient work had been done on finding thermophilic enzymes, even though they were "crucial to establishing a full high-temperature process of vaccine manufacture", adding: "As the patent lawyers have advised, [xxxxx xxxxx xxxx xxxxx xxxxx xxxxx xxxxx xxxxxx xxxxxxx xxxxxx xxxxxx xxxxx]" The note went on to refer to the work that was being done using mesophilic enzymes. There was no suggestion here, or in a list of points she raised for discussion at the board meeting on 7 July 2009, or in the minutes of that meeting, that Dr Hill regarded that work as being something which TGL was not entitled to undertake.
292. Mr Ohlson responded on 16 February 2010, saying:
"A PCT application for patent 2 entitled "Production of closed linear DNA" has been filed. We believe that all the claims in this patent have been substantiated by scientific data developed between May and December 2009. The patent application covers widest range of physical conditions for carrying out the process including temperature. Our patent attorneys inform us that Dr Vanessa Hill has been declared as the only inventor on the PCT application. The application will be published in six months time (six months from the 31st January 2010)."
He went on to explain that a PCT application had not been filed based on the "DNA amplification" application and that it had not been possible to achieve satisfactory thermophilic DNA amplification. |
Accepted in relation to paragraph 288 - this is advice given by Kemps the publication of which could possibly be damaging to Touchlight and redaction would not materially affect the ability of the public to understand my reasoning.
Rejected in relation to paragraph 292 - I am unable to understand how publication of these passages could possibly damage Touchlight. The material in the second passage is in any event apparent from the PCT application. |
|
Limbs 2 and 3
307. It was put to Mr Ohlson that his evidence of reliance related solely to the board meeting of 2 December 2008. It was then put to him that the only application that had been drafted at that time was the one relating to improved DNA amplification, in which claim 1 related to an amplification process carried out under thermophilic conditions. The suggestion was that therefore the board could not have been relying on any representation by Dr Hill as to TGL's rights in processes which were not carried out under wholly thermophilic conditions.
308. I do not accept that. First, while claim 1 of the draft application relating to improved DNA amplification did specify that the amplification step should be carried out under thermophilic conditions, the description made it clear that other steps, including cutting the concatemers, could be carried out at any temperature. Secondly, Mr Ohlson made it clear that his evidence did not relate solely to what happened at the board meeting on 2 December 2008. Indeed, there was a meeting of the directors of TGL on 30 January 2009, following Dr Hill's email of 29 January, at which it was recorded that patent 1(b) had been completed for filing the same day.
313. Mr Ohlson also said that, had the board of TGL believed that Dr Hill had an issue with the intellectual property position, it would have done whatever it sensibly could to remedy any issues including seeking a confirmatory assignment from Dr Hill. Ms McKechnie confirmed that by a confirmatory assignment Touchlight meant an assignment for no or nominal consideration. Kemps advised seeking such an assignment from the inventor(s) as a matter of course following filing of the priority application but it appears that no such assignment was sought from Dr Hill, at least until the national applications were being filed in late 2011. However, if Dr Hill had raised an issue about ownership of the inventions before early 2012, there is no basis for thinking that she would then have been prepared to execute such an assignment. Indeed, all the evidence suggests that she would have demanded significant value for an assignment if the issue of ownership had been live. In my judgment, the loss of the chance to seek a confirmatory assignment is theoretical rather than real. |
Rejected. As to paragraphs 307 and 308, I do not see how publication of this high level material relating to a draft patent application from 2008 could be damaging to Touchlight. As to paragraph 313, see my comment on paragraph 185 above, and the second part of the sentence is of some significance in dealing with the argument advanced by Touchlight. |













