DECISION
Introduction
1. The Appellant, L R R&D LLP (the "LLP"), appeals against closure notices issued by HM Revenue and Customs ("HMRC") which disallowed partnership losses totalling £14,715,954 (comprising £3,656,741 in 2014-15, £11,055,590 in 2015-16 and £3,623 in 2016-17).
2. HMRC contend that the LLP's losses arose pursuant to a tax avoidance scheme in which the LLP was used to deliver the scheme's purported benefits, ie creating losses which could then be claimed by the members of the LLP to set off against other income.
3. The LLP contends that, although the arrangements concerned involve a number of agreements and several different parties (as described below), it is, in essence, entitled to claim the losses which arise from expenditure it incurred under an agreement, dated 25 March 2015, that it entered into with NPL Sub-Contractor Limited ("NPL-Sub"), described as a research and development framework agreement (the "Framework Agreement"), for research and development ("R&D") to be undertaken in respect of rights that the LLP had acquired as part of its trade, and that the tax deductible amount was increased on the basis that the expenditure qualified for R&D Relief under Part 13 of the Corporation Tax Act 2009 ("CTA 2009").
4. Although the LLP made a total of £8 million in payments, it accepts that as only £2.9 million of R&D has actually been delivered, any claim for relief should be restricted accordingly.
5. Rory Mullan KC appeared for the LLP. HMRC was represented by Imran Afzal, Harry Winter and Riya Bhatt. We have been greatly assisted by their submissions, both written and oral. Although we have taken all of them into account, we have not found it necessary to make specific reference in our decision to all of the submissions, materials or authorities to which we were referred.
Evidence
6. In addition to an electronic hearing bundle comprising 3,890 pages, we heard from Dr Fazlul Chowdhury and Mr Bashir Timol who gave evidence for the LLP. No witnesses were called for HMRC.
7. Dr Chowdhury described himself as a "technologist", someone who looks for problems and finds technological and scientific solutions to them. He is the Chief Executive Officer ("CEO") of Nemaura Pharma Limited ("NPL"), a private pharmaceutical company, for which, in addition to being its chief scientist and innovator, he deals with licensing, partnerships and inward investments. Dr Chowdhury is also CEO of Nemaura Medical Inc ("NM"), a company incorporated in Nevada, USA and publicly listed on the Nasdaq stock exchange. He has around 130 patents across multiple platforms.
8. Mr Timol, although not a scientist or tax adviser, has, he said, "experienced success in identifying financial opportunities and bringing together people who are specialists in their relevant field to make an entrepreneurial concept into a reality." He is the director of NPL (from January 2007) and OneE Group Limited ("OneE") (from October 2007). OneE provides tax advisory services, particularly in regard to R&D. In addition to NPL and OneE, Mr Timol is a director of a number of other companies in, what he described as, the "tax field". He explained his role at OneE is to "look after the various stakeholders" which includes "keeping them informed and engaged on the R&D which is taking place." He accepted that he was "a controlling mind" of the LLP.
9. Both Dr Chowdhury and Mr Timol were criticised by Mr Winter for inconsistencies in their evidence.
10. In relation to Dr Chowdhury, Mr Winter criticised his reference to an "eightfold return" for investors in the LLP. Other inconsistencies highlighted by Mr Winter included:
(1) Dr Chowdhury's references to the funding of the LLP. In his witness statement he had said that the LLP was only funded to "a certain stage pre-clinical", but orally in evidence claimed that he did not know the extent to which it was funded;
(2) the fact that Dr Chowdhury had said in his witness statement, and confirmed when giving evidence, that NPL had obtained "individual, independent market research for each and every molecule" when that was not the case; and
(3) Dr Chowdhury's claim that there was an outline for the development pathway in the business case, from the beginning of the concept through to the end of development, for "all" of the molecules when there was no such outline for lidocaine, one of the molecules with which this appeal is concerned (the other being risperidone).
11. Mr Winter submits that Mr Timol's inconsistencies in his evidence include:
(1) his references to an "eightfold return" for investors;
(2) what he said in respect of the provision of quotations from third party R&D providers to OneE;
(3) that a "bona fide" benchmarking exercise had been undertaken by NPL rather than NPL-Sub;
(4) his references to the ratio of fees in the £8 million raised by the LLP; and
(5) his acceptance during cross-examination that one purpose of the £8 million payment by the LLP to NPL-Sub was for the LLP to secure tax relief for its investors, something he "corrected" when re-examined when he said that it was the members investing in the LLP that wanted the tax advantage and that the business of the LLP was to "conduct R&D work."
12. Mr Timol also accepted that as an investor in NPL he could "see that argument" when it was put to him (by Mr Winter) that he had "a slightly conflicted position" when he was trying to get the best deal for the LLP while also having a commercial interest in the main or proposed R&D provider.
13. Additionally, Mr Winter contrasted what had been said by Dr Chowdhury and Mr Timol in evidence. For example, in relation to providing quotations to OneE from third party R&D providers, Mr Timol maintained that the process was "bona fide" and "done properly", whereas Dr Chowdhury had said, "it was actually not possible to go out there and get a realistic quotes", explaining, when it was put to him (by Mr Winter) that "the quotes weren't realistic", that "it would not have been possible to go out there and ask somebody to exploit our [NPL's] technology platform without us handing over our IP."
14. Dr Chowdhury had also said that the purpose (not the "only purpose" as Mr Winter submitted) of providing quotes "was to provide evidence to OneE that we were actually competitive and that these were in tune with what the industry norms were in terms of the charges that are levied."
15. Another example of an inconsistency between Dr Chowdhury and Mr Timol to which were referred by Mr Winter was that Dr Chowdhury considered that the drugs [lidocaine and risperidone] at the time they had been selected "could be developed successfully", whereas Mr Timol accepted there was "a high risk of some of the drugs not working out."
16. Notwithstanding this criticism, we found both Dr Chowdhury and Mr Timol to be straightforward and credible witnesses, especially as both had prepared their own witness statements without any assistance. Dr Chowdhury explained that when he was asked to prepare a witness statement nothing had been scripted, he was just told to "[t]ell us what you did." Similarly, Mr Timol said that he was asked to prepare his witness statement setting out his recollections and "that's what I did."
17. We also agree with Mr Mullan that some of the differences between Dr Chowdhury and Mr Timol may have arisen because of the way in which questions were put to them. For example, Mr Winter asked both Dr Chowdhury and Mr Timol about the likelihood of success of the drugs. In relation to Dr Chowdhury the transcript records:
"Q. You are saying that there was a reasonable likelihood that both drugs would be successful; yes?
A. We set out with those drugs with a view that we felt at the time they could be developed successfully, that's correct."
However, Mr Winter put the question differently to Mr Timol, as recorded in the transcript:
"Q. It was extremely unlikely that both drugs would be successful, wasn't it?
A. Yes, there was a high risk of some of the drugs not working out, I accept that, yes."
Approach to Evidence
18. At [15] - [20] in Gestmin SGPS SA v Credit Suisse (UK) Ltd & Anor [2020] 1 CLC 428, Leggatt J (as he then was), in his oft-cited comments on the unreliability and fallibility of the human memory and the adverse effect that the process of civil litigation has on it, said, at [22]:
"In the light of these considerations, the best approach for a judge to adopt in the trial of a commercial case is, in my view, to place little if any reliance at all on witnesses' recollections of what was said in meetings and conversations, and to base factual findings on inferences drawn from the documentary evidence and known or probable facts. This does not mean that oral testimony serves no useful purpose - though its utility is often disproportionate to its length. But its value lies largely, as I see it, in the opportunity which cross-examination affords to subject the documentary record to critical scrutiny and to gauge the personality, motivations and working practices of a witness, rather than in testimony of what the witness recalls of particular conversations and events. Above all, it is important to avoid the fallacy of supposing that, because a witness has confidence in his or her recollection and is honest, evidence based on that recollection provides any reliable guide to the truth."
19. However, in Kogan v Martin & Ors [2019] EWCA Civ 1645 at [88], Floyd LJ, giving the judgment of the Court of Appeal, made it clear that Legatt J's statements in Gestmin was not an "admonition" against placing any reliance at all on the recollections of witnesses and that:
"... a proper awareness of the fallibility of memory does not relieve judges of the task of making findings of fact based upon all of the evidence. Heuristics or mental short cuts are no substitute for this essential judicial function. In particular, where a party's sworn evidence is disbelieved, the court must say why that is; it cannot simply ignore the evidence."
20. Adopting such an approach, we turn to our findings of fact.
Facts
21. NPL was established by Dr Chowdhury in 2005. It is a company that specialises in pharmaceutical research and development and has (in conjunction with the Nemaura Group) has developed and secured a range of pharmaceutical technologies that are pending and/or granted by UK and international patents. Dr Chowdhury, in evidence, said that NPL had the knowledge, skills, ability and capacity to undertake R&D "in house" from the basic concept through to the end product at its laboratories. The only exception to this being "instances" if it does not have a very specialised and specific piece of equipment that is required. In such circumstances, Dr Chowdhury explained, it is necessary to consider whether such equipment should be purchased or an outsource service provider engaged.
22. Dr Chowdhury also explained that, as is standard across the pharmaceutical industry, it was necessary to outsource clinical trials and that it was often a regulatory requirement for a clinical study to be independent of the R&D company.
23. Following a reorganisation of NM, in 2013 NPL was looking for new investors to finance the development of what Dr Chowdhury described as , a "large number of assets" it wanted to exploit commercially. These included the licencing and development of transdermal patch technology to deliver generic drugs. Having previously dealt with Mr Timol and OneE, which had invested in NPL from 2006, Dr Chowdhury turned to them again and was told that they would be able to raise a substantial amount of money through investors using "tried and tested methods" to structure companies in such a way which would benefit the investors. Mr Timol also accepted during cross-examination, although he rowed back from it in re-examination, that one purpose of the payment to NPL-Sub was for the LLP to secure tax relief for its investors.
24. Such arrangements, it was said, would allow NPL to operate and retain ownership and therefore control over its intellectual property and, as Dr Chowdhury explained, allow him to develop the products much faster than if he had to spend time trying to raise external investment and find the right group of investors.
25. The proposed structure was to establish an LLP in respect of a set of molecules, with investors for each LLP. In all, there were 11 LLPs, all of which entered into the same arrangements under which a licence for the exploitation of the transdermal patch technology was granted to the LLP by NDM Technologies Limited ("NDM"). Mr Timol explained, in evidence, that:
"... the whole thing is incentivised by the tax structure, there's no question of that, I'm not denying that. But that can't detract from the underlying substance which is research and development into clinical molecules, into you know medicines. That is the substance of what is happening here, and there's no - to my mind subtracting away from that."
26. It was anticipated that through R&D, the LLPs would develop the rights licenced to them and exploit them for profit. Although the R&D has been successful, the market has changed and neither the LLPs nor NPL has, to date, managed to sell the developed product.
27. The present appeal, as noted above, is that of the LLP and concerns the molecules Lidocaine and Risperidone. It involves a number of contemporaneous agreements (described in greater detail below) that confer rights and obligations on the LLP. Appeals of the ten other LLPs which, although concerning different molecules, are, in factual and legal terms, materially identical to the present appeal and are stood behind it.
Information Memorandum
28. On 5 March 2015, Prosper Capital LLP ("Prosper"), in its capacity as sponsor appointed by the LLP under the terms of the Sponsor and Operator Agreement (see below), issued the Information Memorandum ("IM").
29. After stating its contents should not be treated as "advice", recommending investors "consult an independent financial advisor" and warning of the potential exposure to "significant risk" to a potential investor, that they could lose "some or all of your capital, and potentially more than your capital if you are required to contribute to the losses of the [LLP] pursuant to the Partnership Deed", the IM continues:
"This Information Memorandum is issued by the Sponsor and relates to an opportunity to participate in the [LLP], which is a UK limited liability partnership that has been formed to engage in the trade of exploiting patent rights by undertaking research and development and bringing to market drugs and other pharmaceutical molecules with patented drug delivery technologies. This Information Memorandum sets out the terms on which corporate entities may become members (each a "Member") of the [LLP] (the "Opportunity").
30. The Executive Summary (Part 1) of the IM, under the sub-heading "The technologies", explains how in healthcare, transdermal (into the skin) drug delivery is becoming increasingly accepted as an effective means of painlessly delivering drugs to patients. It also identifies and gives examples of a number of issues with this process.
31. These include:
(1) the limitation on the number of drugs that can be delivered through the skin because of the physical size of the drug molecules;
(2) that larger doses need larger patches which are harder to apply to the skin and therefore to ensure that the correct dose is administered;
(3) that patches use pressure-sensitive adhesives to attach to the skin which in many cases lead to skin irritation and fall off;
(4) that the depth of penetration of the micro-needle will vary from person to person;
(5) that expensive actuators are needed to force the drug into the skin;
(6) that arrays of needles are required to allow the dose to be spread and give rapid absorption of the drug but if one or more of the needles leaks it leads to back flow;
(7) that the pressure across the needles may become uneven leading to inaccurate dosing; and
(8) that multiple needles on an array means the insertion force is spread over the needles needing high forces to ensure all the needles enter the skin.
32. The IM explains that the Nemaura Group, of which NPL is a member, has sought to address these problems by developing patches (the "Patches") using micro-needles to deliver a range of drugs into the skin and that NPL is conducting tests on a number of drugs to assess whether they can be successfully delivered using the Patches. It also describes the benefits of such an approach, and explains that the Nemaura Group has a number of patent rights "granted and pending" covering the Patches and that the core patents covering the technologies has been published in a number of countries including the UK, US, Canada, China and Japan.
33. After stating that further patents covering further aspects of the technology are in earlier stages, the IM continues:
"The Nemaura Group believes there is the potential for a wider range of drugs to be delivered using each of the Patches and has commenced testing on the compatibility of the technologies with a number of drugs. However, each drug will require extensive testing before it can be delivered commercially using a Patch. The Patches may also require customisation to work with different drugs. The Nemaura Group believes that significant research and development funding will therefore be needed to achieve the commercial potential of its Patch technology with a wider range of drugs.
The Nemaura Group has indicated that it is willing to grant patent rights under [the] licence for research and development purposes for the [Patches] in connection with the drugs lidocaine and risperidone. The [LLP] intends to profit from the development of the Patent Rights to deliver the Molecules commercially to patients (the "Venture")."
34. The "Opportunity Review" (Part II) of the IM sets out the proposed arrangements. It states that the LLP is seeking total capital of up to £2,080,000, that members may be admitted by the LLP on a periodic basis and that prospective members are offered the opportunity, subject to the Application Form, to make capital contributions to the LLP of at least £10,000 and up to a maximum of £1 million per member.
35. The "Strategy Overview" explains that the LLP:
"... will, pursuant to a Licence Agreement, obtain the right to exploit the compatibility of the Molecules with the Patent Rights obtained. The Licence Agreement, subject to conclusion of any negotiations, is expected to be entered into on or around 16th March 2015.
36. After summarising the intended agreements to be entered into, the IM, under the sub-heading "Assessment and Success", explains that during the early stages of R&D the LLP will assess the likelihood of Patches being commercially successful, with success being assessed at various stages according to different "milestones" being met. The IM describes how, if the LLP assesses that there is a prospect of success in one or both molecules, ie in this case, lidocaine and risperidone, it may seek to dispose of the technology that had been developed in which case NPL could, at its discretion, exercise its right under the Pre-emption Deed (see below) to acquire that technology from the LLP.
37. If the LLP did not dispose of the technology the Joint Venture Agreement (see below) will provide that the receipts of the exploitation of the technology will be shared between the LLP and NPL and that the LLP will distribute the profit in accordance with the partnership allocations as set out in the Partnership Deed (see below).
38. The IM also explains that testing against a particular molecule may cease if continued testing on that particular molecule is no longer commercially justifiable. It also explains what will happen to the funds and how the third party loan will be repaid.
39. The "Opportunity Overview" section of the IM concludes with an indicative timetable, giving the last application date as 23 June 2015 with a closing date of 26 June 2015. It also sets out the "Application Procedure" for prospective members who are required to remit to the Sponsor, ie Prosper, a completed Application Form, funds representing the relevant capital contribution and anti-money laundering documentation.
40. The next section of the IM (Part III) is headed "Risk Factors".
41. It explains that joining the LLP "involves a high degree of risk" and that it is suitable only for those corporates that "fully understand and are capable" of bearing such risks and warns that only those corporates able to "sustain a total loss to their capital" should apply to join the LLP. The IM also warns that the returns to members will be dependent on the financial performance of patent rights and product with a molecule and that the assumptions and targets included in the IM "are illustrative only and may not be achieved in practice." In addition, it warns that R&D is "inherently risky" and that it is not possible to accurately predict the final value of the product, the fees that can be achieved or the prospect of success of the testing.
42. Under the sub-heading "Innovation risks", it explains that although the LLP in conjunction with NPL has identified the molecules, in this case lidocaine and risperidone, as having the "potential" to be commercially delivered using the Patches there is no guarantee that the venture will succeed and, as has proved to be the case, it might not be possible to achieve a commercially viable product.
43. There is also a warning that as the LLP intends to conduct its business in the competitive pharmaceutical R&D market, it will be subject to "usual market, financial and economic conditions" which may change and, due to circumstances beyond the LLP's control, it might suffer a loss and/or the returns to its members may be adversely affected.
44. In addition, potential members are warned that in order to fully fund the R&D the LLP itself "will be leveraged" with the patent rights and products financed by an element of debt which may rank ahead of any revenues to be paid to members.
45. The "Risk Factor" section of the IM concludes with the following general warning:
"The Venture described in this Information Memorandum is not suitable for everyone. Potential Members must rely on their own examination of the legal, taxation, financial and other consequences of participating, including the risks involved, and must consult their own independent financial advisers, including an adviser authorised under FSMA."
46. In Part IV, the IM sets out the Parties to it and its Advisors. The Sponsor and Operator is Prosper and OneE is the "Asset Arranger". Prosper and OneE are both "Initial Designated Members" of the LLP.
47. The next section of the IM (Part V) "The Company & The Molecules" contains a brief description of NPL and Dr Chowdhury before setting out the background and market research undertaken by Roots Analysis (see below) in relation to the molecules, Lidocaine and Risperidone. This is followed by a "Technical Summary" of those molecules that explains, in broad terms, the areas that need to be considered in relation to skin infusion studies with micro-needles that have been undertaken.
48. Part VI of the IM, "The Partnership", sets out the initial structure of the LLP, how profits and losses are to be allocated as well as describing the LLP's termination. It also explains that once the amounts of the aggregated capital contributions have been calculated, the LLP intends to obtain borrowing, "equivalent to 74% of the total" funds, from a third party lender.
49. Part VII of the IM, "Partnership Activities", explains that the LLP has been established for the purpose of carrying on the "Trade" which it intends to commence as soon as it has been "capitalised, in the manner contemplated" by the IM. It continues by identifying the venture as obtaining the patent rights and bringing to market the product.
50. In terms of R&D, the IM states:
"As the [LLP] itself does not currently have any employees, it expects that it will be necessary to sub-contract the provision of the majority (if not all) of the Research and Development to one or more Sub-Contractors.
In order to provide the necessary commercial certainty that the Research and Development will be undertaken by the [LLP], it is the [LLP's] intention to forward-purchase the necessary Research and Development from the relevant Sub-Contractors under Research and Development Sub-Contracts shortly after entering into the Licence Agreement.
As such, the [LLP] expects to have to pay each Research and Development Sub-Contract price in full to the relevant Sub-Contractor on or shortly after entering into the relevant Research and Development Sub-Contract."
51. This section of the IM concludes with the following table illustrating the projected returns if both Molecules are successful.
|
RESULTS (YEAR) |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
TOTAL |
|
Partnership profit/loss (£m) |
-8 |
0 |
2 |
0 |
4 |
0 |
3.4 |
8.2 |
9.6 |
52. These results are based:
"... on the assumption that the [LLP] raises the Maximum Total Capital and that following the early stages of the testing, [NPL] exercises their pre-emption right to acquire the Developed Technology covering those Molecules and milestone payments are paid by a pharmaceutical company with a view to the pharmaceutical company bringing the Product to market."
53. However, in a preceding paragraph, but on the same page as this table, the IM warns that:
"Financial illustrations and projections contained in this Information Memorandum are for illustrative purposes only. Nothing in this Information Memorandum should be construed so as to constitute a forecast return and none should be implied or inferred. ..."
54. For completeness, we should mention that Part VIII of the IM contains a summary of costs and fees, Part IX summarises the details of the LLP and Partnership Deed (for which see below) and Part X provides details of the LLP's material contracts. The IM concludes, at Part XI, with a glossary of defined terms.
Seminars/Roadshows
55. Once it was completed, the IM was issued to potential investors by OneE. Dr Chowdhury said that these numbered "hundreds, if not thousands" of accountants and contacts representing high-net worth individuals seeking to invest in "these type of opportunities."
56. Dr Chowdhury and representatives of OneE also arranged seminars or "roadshows" across the UK. These were, Dr Chowdhury explained, structured in two halves. The first, presented by OneE representatives was entirely about the tax structure and its operation, albeit with a "health warning" that it was always possible that it might be challenged by HMRC. The other half was based on the technology and was presented by Dr Chowdhury.
57. The presentations explained that each drug would take "around" three years to test and would require "between £2 - £4m of investment depending on the drug". Potential investors were also told that should the drug pass testing, NPL would "acquire the technology from the LLP at around 7 times [the] original investment." The roadshows would conclude with a question and answer session. Dr Chowdhury said that those who attended were extremely engaged, despite the fact that many of them, being business people of all types, did not have a science background.
58. In addition to the roadshows, NPL hosted open days at its laboratories in Loughborough for the prospective investors and their representatives at which further question and answer sessions were held.
Market Research
59. Independent market research reports were commissioned from Roots Analysis Private Limited ("Roots") in relation to 32 drugs (and not "each and every molecule" as Dr Chowdhury said) to identify whether there was a market that justified the R&D being undertaken.
60. Taking the report, received in 2015, in relation to lidocaine, "Lidocaine, Delivery via Microneedles", as an example, its "Executive Summary" explains that Lidocaine is a "local synthetic anaesthetic" and that:
"Topical patches, Lidoderm (Endo Pharmaceuticals) and Synera (Galen US) have been approved for treatment of post herpetic neuralgia and local dermal analgesia respectively."
61. The Executive Summary continues by setting out the "Competitive Landscape" noting that:
"● The primary competition comes from other local anaesthetic drugs such as articaine, bupivacaine, mepivacaine and prilocaine.
● Apart from the competition from other APIs, topical patch (lidoderm patch 5%) also faces stiff competition from several generics that are available in various markets in and outside the US.
● In future, additional competition for lidocaine is likely to come from drugs which are currently under development for topical administration; examples include Fortacin, T2380, PeriZone PerioPatch etc."
62. The Executive Summary then refers to "Current Challenges and Advantages of Microneedle Delivery", observing it is a "well-established challenge" that for the topical patch formulation less amount of drug absorption takes place. Other challenges include the accidental falling off of the patch from the skin and possible allergic reactions. With regard to the "Commercial Potential", the Executive Summary records the decline in sales of lidocaine with an expectation that the market would stabilise by 2030. It also noted that a successful launch of NPL's microneedle product had the potential to capture a "moderate to high share of this market", which in a "particular scenario" at a 35% share NPL could:
"... look to gain ~USD 10 million as upfront payments, ~USD 70 million as milestone payments in addition to royalty payments on future sales."
63. The Lidocaine report also included a section containing a detailed analysis of the benefits of, and approach to, the delivery of drugs via microneedles as well as an "Opportunity Analysis".
64. Material sections of that "Opportunity Analysis" state:
"8.1 Methodology
The approach, outlined below, is based on estimating the valuation (NPV) of a licensing deal for both [NPL] (licensor) and the partner (licensee). The extent of valuation shared between [NPL] and the partner will define the limits for upfront, milestone and royalty payments. However, each of these individually can be flexed to get to the same return for [NPL] under different scenarios.
The NPV of the partner will be primarily hinged on the following two parameters:
1. Incremental Revenue, from delivery of drug via the new technology
2. Costs, which will come in three categories.
a. Pre-launch R&D and marketing
b. Ongoing CoGS, SG&A
c. Payments made to [NPL] (Upfront, Milestone, Royalty)
For [NPL], the NPV is based on the following:
3. Future Revenues, same as Point 2c above
4. Costs, which represents total money already spent on technology development.
...
8.3 Cost Drivers
In order to get the product in the market, additional expenses will be incurred on research & development, gaining market approval, pre-launch marketing etc. We have assumed that NPL's partner will incur 100% of this expense as may be agreed in the terms of the deal. Post launch, ongoing CoGS and SG&A expenses will also have to be considered."
65. The report concludes with the following:
"DISCLAIMER
This report presents work done by analysts at Roots Analysis Private Limited. The views expressed in this report represent independent opinion of the analysts. These views are formed based on extensive business research and are published solely for guidance and information purposes. This report is not a substitute for tailored professional advice. These views shall not be misconstrued as a firm guideline for taking business decisions. We make no warranties as to the accuracy or completeness of information and opinions contained herein. This report may not be sold without the written consent of Roots Analysis."
66. When asked about this, Dr Chowdhury dismissed it as the "standard disclaimer" that any such organisation puts in its reports. However, he was of the view that it would not be "really sensible" to make a business decision solely on the basis the report and that this had not been done in the present case where, as he said in evidence, there were:
"... a whole host of documents, there were discussions, there were lab tours, there were demonstrations, there were a whole host of activities that were undertaken before even we as a company determined that we should pursue certain developments, because ultimately what are we - what was it we wanted after at the end of this? We wanted products to reach market and so that we could generate income."
67. A similar Roots report was obtained in relation to risperidone. The final paragraph of the Executive Summary of that report, which reflected the fact that risperidone is an anti-psychotic drug with a very different market from lidocaine, a local anaesthetic, noted:
"● Due to the unmet need of self-administered injectables (resulting in in high non-compliance), the microneedle based product from [NPL] has the potential to capture moderate / high share of this market. In one particular scenario, at a 35% share of total pre-tax valuation, [NPL] could look to gain between USD 5-10 million as upfront payments, USD 20 - 30 million as milestone payments in addition to royalty payments on future sales."
Agreements
68. There is no issue between the parties that the following agreements were executed by the relevant parties and that some key personnel were involved in more than one agreement.
69. Turning to the various agreements:
Sponsor and Operator Agreement
70. The Sponsor and Operator Agreement was entered into on 5 March 2015 between Prosper, as a person authorised by the FCA to undertake certain functions on behalf of the LLP, OneE and the LLP. As noted above, the LLP appointed Prosper, under this Agreement, to act as sponsor of the IM and operator of the LLP, and to provide or procure the provision of the services set out in Schedule 1.
71. The Sponsor Services included reviewing and issuing the IM, and the Operator Services included arranging the preparation of the LLP's accounts and maintaining or procuring the maintenance of a register of the interests of members in the LLP. There was to be a Sponsor of £11,500 plus VAT on the date of each Partnership Close (the final date in a calendar month on which members would be admitted to the LLP), and it was intended that there would be monthly Partnership Closes until the LLP was fully funded. The Operator Fee was a sum equal to 1.25% of the distributable profits of the LLP after the LLP's tax liability due on each anniversary of the Partnership Close until the LLP was wound up. The Operator services included an obligation on Prosper to:
"Maintain or cause to be maintained for the purposes of any obligations under FSMA the [LLP's] records and books of account and procure that such records and books of account are maintained to reflect the activities of the [LLP]."
LLP Partnership Deed
72. The parties to the Partnership Deed, dated 23 March 2015, are OneE (as the "First Founding Member"), Prosper (as the "Second Founding Member") and the LLP (as "the Partnership"). Mr Mullan described many of the clauses in the Deed as "boiler plates" and, as such, these are not set out below. However, it is necessary, as part of the context and background to the arrangements entered into by the LLP to refer to several clauses of the Partnership Deed.
73. Various definitions are contained in Clause 1. These definitions include:
""Designated Members" means those Members who are from time to time designated for the purposes of section 8 of the [Limited Liability Partnership Act 2000] in accordance with Clause 7.1 (Number and identity of Designated Members).
"Drug" means a particular drug or medication in relation to which the Partnership has obtained a licence to exploit the TDP Technology as a means of delivery for.
"Fundraising Accounting Period" means an Accounting Period
(a) during which an Introducing Subscribing Member makes a capital contribution to the Partnership; and
(b) which is designated as such by a Designated Members' Decision issued by the Designated Members.
"Know-How" means the unpatented technical and other information relating to the TDP Technology and which is not in the public domain regardless of how such information is collected or recorded, and whether protected by copyright, design rights or otherwise and including without limitation, all inventions, data, designs, formulae, compounds, methods, models, assays, research plans, procedures, results of experimentation and testing (including results of research and development) data analyses, reports and information contained in any submission to ethical committees and regulatory authorities.
"Introducing Subscribing Member" means in respect of any single Accounting Period a Subscribing Member who makes a capital contribution in that Accounting Period under agreement with the Designated Members, of not less than £10,000.
"R&D Agreement" means any agreement for the provision of research and development services in connection with the use of TDP Technology as a means of delivering a drug to the bloodstream of a patient.
"Subscribing Member" means all persons who are admitted to the Partnership as a Subscribing Member
"TDP Technology" means the transdermal patch technology for delivery of medications to the bloodstream of a patient, the Know-How and the patents and patent applications relating to it, ..."
74. Clause 2 provides that the LLP is subject to regulation and requires an Operator to establish, operate and in due course wind up the LLP.
75. The nature and purpose of the LLP is set out at Clause 3. The material parts of Clause 3.2, headed "Purpose" provide:
"3.2.1 Without prejudice to the powers (at law or otherwise) of the Partnership to carry on any other business from time to time, the purpose of the Partnership is to carry on the Trade (as defined in Clause 3.2.2).
3.2.2 the Partnerships trade (the "Trade") shall be the exploitation of the TDP Technology with a view to profit and shall include:
3.2.2.1 the acquisition of rights to exploit the TDP Technology in relation to a given Drug under a License Agreement ("acquired rights");
3.2.2.2 the enhancement of the value [of] acquired rights through the carrying out of relevant research and development to ascertain and/or establish the viability of the TDP Technology with a given Drug
3.2.2.3 the sale and/or further licence of the acquired rights; and
3.2.2.4 the engagement by the Partnership in any matter, service or enterprise that is ancillary or related to the Trade, or which the Partnership considers desirable in connection with the Trade
3.2.2.5 the entering into agreements by the Partnership with any other party in pursuance of the above.
76. The number and identity of the "Designated Members" are set out at Clause 7. This provides that at all times no fewer than two Members are Designated Members (Clause 7.1.1) and that the Designated Members shall be, as at the date of the Partnership Deed, the First Founding Member (ie OneE of which Mr Timol is a director) and the Second Founding Member (ie Prosper).
77. Clause 11 of the Deed provides for the allocation of profits and losses. It distinguishes between "Fundraising Accounting Periods", in which an Introducing Subscribing Member made a capital contribution designated as such by the Designated Members, and other "Accounting Periods". In an Accounting Period "other than a Fundraising Accounting Period" Clause 11.1.1.1 provided:
"100% of Net Income, Net Losses, Capital Gains and Capital Losses to the Subscribing Members allocated by reference to their respective Subscription Proportions in the Contribution Period in which the Accounting Period falls."
78. The allocation for a Fundraising Accounting Period, was, as stated in Clause 11.1.2:
"11.1.2.1 As to 0.2% of Net Income, Net Losses, Capital Gains and Capital Losses to the Subscribing Members who are not, in respect of that Fundraising Accounting Period, Introducing Subscribing Members, in proportion to the cumulative total of each such Member's Subscriber Contributions as recorded in the Register of Capital Contributions
11.1.2.2 As to 99.8% of Net Income, Net Losses, Capital Gains and Capital Losses to the Introducing Subscribing Member or Introducing Subscribing Members and, if more than one, in proportion to their respective capital contributions made in that Accounting Period."
Licence Agreement
79. The Licence Agreement between NDM, NPL and the LLP was executed on 23 March 2015.
80. In its recitals, under the heading "Background", it is stated:
(A) NDM is an affiliate of [NPL], and exists as an Intellectual Property Rights holding company for [NPL] and its Affiliates. NDM holds the patented Technology as a means for delivering medications through minimally invasive means.
(B) The viable use of the Technology as a means of delivering the Drugs has not yet been established.
(C) NDM intends to grant the LLP an exclusive licence to exploit the use of the Technology as a means of delivering the Drugs.
(D) [NPL] shall, on behalf of itself and NDM, be responsible for liaising with the LLP on behalf of [NPL], NDM and, if relevant, their Affiliates, and for making any applicable decisions concerning the exercise of its and NDM's rights and obligations under this Agreement.
81. The definitions, contained in Clause 1, include the following:
"Affiliate In relation to any company, a subsidiary of that, company or a holding company of that company (as each such relevant term is defined in the Companies Act 2006) and any other person or entity which is associated to that company. Associated carries the same meaning as the Corporation Tax Act 2010 s 25(4).
Confidential Information: Any and all information and materials of a commercially sensitive or secret nature, in whatever form, whether tangible or intangible, including any copies, and whether disclosed before or after this Agreement, which is now or at any time after the date of this Agreement owned or controlled by one party, including all summary reports and analyses made by any party or their respective advisors which contain or reflect such information notwithstanding the information is owned or confidential to a third party, whether technical, commercial, financial or otherwise, relating to that party and/or its products, intellectual property rights, business or marketing activities, and which is marked confidential and/or from the circumstances in which it is made available to the other party the information ought to be treated as confidential.
Completion of Research: Either (i) all of the research specified in Appendix 2 has been carried out in relation to that Drug; or (ii) all such research and development as might reasonably be considered necessary to enable the Technology as it relates to a particular Drug to be lawfully marketed on a commercial basis; or (iii) the R&D Firm has informed the LLP that use of the Technology in relation to that Drug is not commercially viable.
Effective Date: The date of this Agreement.
Drug 1: Lidocaine - local anaesthetic.
Drug 2: Risperidone - atypical antipsychotic.
Drug 3: Porcilis - live attenuated PRRS virus (animal vaccine).
Drug 4: Duramune - multivalent canine vaccine.
Initial Drugs: Drugs 1 to 4.
Drugs: The Initial Drugs and the Supplemental Drugs.
First Pair: Drugs 1 and 2.
Fully Funded: Research and Development is carried out on a "Fully Funded" basis where an R&D Firm:
(a) has contracted to carry out either (i) all that Research and Development specified in Appendix 2 or (ii) all such Research and Development as might reasonably be considered necessary to enable the Technology as it relates to a particular Drug to be lawfully marketed on a commercial basis and
(b) (by the time such Research and Development commences) has been paid by the LLP all undisputed sums due and payable under the R&D Agreement to carry out such Research and Development.
R&D Agreement: An Agreement to be entered into between the LLP and [NPL-Sub] in substantially similar form to the draft Agreement at Appendix 3 (as such terms may be varied by the parties from time to time) or such other written terms as the parties may agree from time to time.
R&D Firm: [NPL-Sub] or any other person or partnership carrying on a business capable of undertaking Research and Development on behalf of the LLP provided that [NPL] has consented in writing (such consent not to be unreasonably withheld or delayed) to the engagement of such person or partnership by the LLP.
Research and
Development Any scientific, technological and/or market research into and/or testing of the Technology as it relates to the Drugs (or any of them) which may include, without limitation,
Research IPR all Intellectual Property Rights generated at any time by or on behalf of the LLP, any R&D Firm or any other sub-licensee in connection with the exercise of the Licence, including any Intellectual Property Rights comprised in or relating to any of the Research Results.
Research Results all technical data, know-how, computer software, notes, chemical compounds, biological material, models, prototypes, specimens, drawings, reports, and information, all documents concerning regulatory submissions and Marketing Authorisations and any improvements to the Technology, generated at any time by or on behalf of the LLP, any R&D Firm or any other sub-licensee in connection with any Research and Development.
Second Pair: Drugs 3 and 4.
Supplemental Drugs: Those four medications and/or drugs which are determined in accordance with Clause 6.
Technology: The transdermal patch technology ... which enables the delivery of medications to the bloodstream of a patient through minimally invasive means and any developments and/or improvements to it developed by or on behalf of NDM and or [NPL] (or any Affiliate of either of them) including the Know How, the Patents and all other Intellectual Property Rights of NDM and/or [NPL] (or any Affiliate of either of them) in any of the foregoing, whether existing at the Effective Date or which come into existence thereafter.
82. Clause 2.1 provides:
"In consideration of the matters set out below (and in particular the benefits to NDM and [NPL] of the Research and Development, and assignment to NDM of the Research IPR and Research Results), NDM hereby grants to the LLP, with effect from the Effective Date, an exclusive royalty-free licence under the Technology to exercise all and any of NDM's rights and privileges anywhere in the world in and to the Technology insofar as it related to each of the Drugs (the "Licence").
83. Under Clause 2.2, NDM (and if applicable) NPL are required, on the written request of the LLP, to make available at no cost to the LLP, "all such documents, records, samples, materials and/or other Know How relating to the Technology ... as are reasonably necessary or desirable" to carry out R&D and/or for the commercial exploitation of the transdermal patch technology. This right has not been exercised by the LLP.
84. Clause 2.3 provides that, without prejudice to the generality of Clause 2.1, the rights granted to the LLP include the use of the Technology insofar as it relates to any of the Drugs (clause 2.3.1), to sub-licence the rights under Clause 2.1 to the R&D Firm (clause 2.3.2) and to manufacture (or procure the manufacture of), dispose of, offer for sale or disposal to any person or evaluate any product which involves the use of the Technology insofar as it relates to any of the Drugs.
85. The parties were also able, pursuant to Clause 2.5, to agree to substitute any one of the Drugs for a different drug.
86. Clause 3 provides:
"3 Research into the Drugs
3.1 The LLP shall, at its own expense, engage the R&D Firm to undertake Research and Development up to Completion of Research to determine the viability of the use of the Technology in relation to each of the Drugs on the terms of the R&D Agreement [ie the Framework Agreement - see below].
3.2 The LLP shall ensure that, within 12 months of the Effective Date, the R&D Firm will have commenced (or procured the commencement of) Research and Development in relation to the use of Technology as it relates to the First Pair, and that such Research and Development shall be carried out on a Fully Funded basis.
3.3 The LLP shall ensure that, within 12 months of Completion of Research in relation to both the First Pair, the R&D Firm will have commenced Research and Development in relation to the use of the Technology as it relates to the Second Pair.
3.4 The LLP will inform [NPL] of the progress of any Research and Development carried out by the R&D Firm from time to time.
3.5 All Research IPR and Research Results shall vest in and be owned absolutely by NDM. The LLP now assigns (and shall precure that each of its sub-licensees, including without limitation the R&D Firm assigns) all of its right, title and interest in and to the Research IPR and Research Results (both present and future) to NDM absolutely.
...
3.8 The parties shall be at liberty to vary or amend (by written agreement) in relation to any Drug the research specified in Appendix 2 to the extent that such becomes necessary or expedient.
3.9 Nothing in this Agreement shall constitute any representation of warranty by the LLP that any Research and Development shall result in any particular outcome.
87. Appendix 2 sets out the areas of scientific and technological uncertainty that require resolution for the transdermal patch technology to succeed in relation to any drug.
Deed of Right of Pre-Emption
88. The Deed of Right of Pre-Emption (or the "Pre-Emption Agreement"), dated 23 March 2015, was entered into between the LLP and NPL.
89. The definitions contained Clause 1, the Interpretation Clause, are in similar terms to those of the other Agreements but also included the following:
"Consideration: The amount payable by [NPL] to the LLP in consideration of [NPL's] acquisition of the Developed Rights following its exercise of the Pre-emption Rights as calculated pursuant to Clause 5.
Disposal The sale, gift, grant, transfer, assignment, novation or any disposal or dealing with the Developed Rights whereby a person other than the LLP would become entitled to all or any of the Developed Rights or any rights deriving therefrom.
Developed Rights: All of the rights which the LLP holds in relation to the Technology as it relates to a Drug, whether such rights exist under the Licence Agreement the R&D Agreement or otherwise.
Pre-emption Event: As defined by Clause 3.
Pre-emption Period: The period of six months after service by the LLP of an Offer Notice on [NPL].
Pre-emption Rights: As defined by Clause 2
90. In addition to defining "Fully Funded" in almost identical terms as in the Licence Agreement (see above), there is also a definition in Clause 1 to "Partly Funded". This provides that R&D:
"... is carried out on a "Partly Funded" basis where the R&D Firm has contracted to carry out Research and Development in relation to the use of the Technology to Deliver a Drug which is not Fully Funded."
91. Under Clause 2.1 the LLP granted NPL the right, following the occurrence of a "Pre-emption Event and at any time during the Pre-emption Period, to acquire the Developed Rights as they related to any Drug which was the subject of a Pre-emption Event, for the Consideration." That right applies to each of the Drugs separately (Clause 2.2).
92. A "Pre-emption Event" is defined by Clause 3. It is only necessary for the purposes of this appeal to refer to Clause 3.1 which provides:
"3.1 If the LLP
3.1.1 receives a genuine offer from a third party to purchase the Developed Rights in relation to one or more Drugs, and the LLP wishes to pursue and/or carry out further investigations and/or negotiations to determine whether or not to make a Disposal of the applicable Drugs on the basis of that offer; or
3.1.2 decides to make a Disposal (as may be evidenced by a letter of intent from the intended purchase if [NPL] so requests) of the Developed Rights in relation to one or more Drugs,
then the LLP shall provide written notice of such occurrence to [NPL], and a "Pre-emption Event" in respect of the affected Drugs shall be deemed to have occurred on the date of such notice."
93. Within five business days of giving notice of a Pre-emption Event, Clause 4.1 requires the LLP had to give NPL an Offer Notice.
94. Clause 5 provides for the Consideration to be paid by NPL to the LLP. Insofar as applicable it provides:
"5.1 If at the date of the Offer Notice either:
5.1.1 all of the research specified in Appendix 2 of the Licence Agreement (or all such research and development as might reasonably be considered necessary to enable the Technology as it relates to the Drug which is the subject of the Offer Notice to be lawfully marketed on a commercial basis) has been carried out in relation to the Drug which is the subject of the Offer Notice; or.
5.1.2 Research and Development is being carried out on a Fully Funded basis in relation to that Drug,
the Consideration for the acquisition of the Developed Rights by Nemaura following its exercise of the Pre-emption Rights shall be the total of the First Cap and the Second Cap.
5.2 Subject to Clause 5.1, if at date off the Offer Notice Research and Development is being carried out in relation to the Drug which is the subject of an Offer Notice on a Partly Funded basis, the Consideration for the acquisition of the Developed Rights by [NPL] following its exercise of the Pre-emption Rights shall be 50% of the Gross Receipts up to:
5.2.1 £100,000; plus
5.2.2 any sums paid by the LLP to the R&D Firm:
(a) in respect of which Research and Development has not been carried out as at the date of the Offer Notice; and
(b) that has not otherwise been refunded to the LLP by the R&D Firm.
5.3 The Consideration ... shall be paid in full by the fifteenth anniversary of the Effective Date ...
5.4 Each Interim Payment shall be calculated as follows:
5.4.1 100% of the Gross Receipts received in the 6 months period to which the relevant Interim Payment relates up to the First Cap; and
5.4.2 once Interim Payments equal to the First Cap have been paid to the LLP, 1/3 of Gross Receipts received in the 6 month period to which the relevant Interim Payment relates to the Second Cap.
5.5 In this Clause, "the First Cap" means:
5.5.1 such amount as is necessary to settle the LLP's liabilities under the Loan Agreement and any Additional Loan;
5.5.2 Plus
(a) if the date of the Offer Notice is before the Second Pair Commencement Date, £2,320,725; or
(b) if the date of the Offer Notice is after the Second Pair Commencement Date, £1,273,150
5.6 In this Clause, "the Second Cap" means:
5.6.1 if the Offer Notice relates to either of the First Pair, the Second Cap shall be £5,137,856 unless [NPL] has already exercised the Pre-emption Rights in relation to the other of the First Pair, in which case the Second Cap shall be £3,419,819;
5.6.2 if the Offer Notice relates to either of the Second Pair, the Second Cap shall be £1,933,720 unless [NPL] has already exercised the Pre-emption Rights in relation to one of the First Pair, in which case the Second Cap shall be £2,761,020;
5.6.3 if the Offer Notice relates to any of the Supplemental Drugs, the Second Cap shall be £1,110,000; or
5.6.4 in the event of simultaneous Offer Notices the Second Cap shall be determined as if Pre-emption Rights had been exercised consecutively in relation to each Drug which is subject to the Offer Notices.
95. Clause 7 makes provision for the failure by NPL to exercise the right of pre-emption either by rejecting an offer contained in an Offer Notice or failing to accept such an offer in which case the pre-emption rights, as they relate to a particular Drug, shall cease and, in such circumstances, it is open to the LLP to dispose of the Developed Rights
96. Clause 8 provides that NPL's right to acquire the Developed Rights in relation to a Drug shall be conditional upon it agreeing that it shall engage the R&D Firm to undertake all such R&D as has been funded by the LLP pursuant to the R&D Agreement which relates to that Drug to the intent that any R&D contracted for and paid for by the LLP in relation to that Drug shall be carried out and that all sums expended by the LLP on R&D in relation to that Drug shall result in a commensurate amount of R&D being undertaken.
Joint Venture Marketing Agreement
97. This Agreement (the "JV Agreement") between NPL and the LLP is dated 23 March 2015 (the "Effective Date"). Its interpretation clause, Clause 1, also contains similar definitions to those seen in the Agreements above, eg Developed Rights, Drug 1, Drug 2 Drug 3 and Drug 4, Technology etc.
98. Clause 2 provides that the agreement shall commence on the Effective Date and continue until 12 months after the latest date on which the LLP enjoyed rights in relation to the Drugs under the Licence Agreement (see above) and would automatically be renewed for a further 12 months unless either of the parties signified their wish to terminate three months before the expiry date.
99. Clause 3, which has the heading, "Sales Strategy", provides:
"3.1 At the request of the LLP [NPL] will draw up a strategy to enable an effective and profitable exploitation of the Technology as it relates to that Drug ("the Sales Strategy").
3.2 The Sales Strategy shall be drawn up in a way which reflects the respective knowledge and expertise of the parties and the respective interests of the parties.
3.3 [NPL] shall have responsibility for implementation of the Sales Strategy.
3.4 [NPL] agrees not to undertake any sales or marketing programme which contravenes the laws of England or any other jurisdiction in which it operates. [NPL] will undertake its sales and marketing business in a legal and decent manner in accordance with the terms of this Agreement and in compliance with any codes of conduct appropriate to the industry.
3.5 The obligations under this clause shall not arise in relation to a Drug if [NPL] acquired the Developed Rights in relation to that Drug pursuant to the Pre-emption Deed.
3.6 If and to the extent that the LLP and/or [NPL] receives or is entitled to receive any amount as the result of exploitation of the Technology as it relates to that Drug, such amount shall be treated as if it were the proceeds of a Sales Strategy whether or not that is in fact the case and shall be shared as provided for in Clause 6."
100. NPL is required, by Clause 4, to act in the "best interests of the parties", to use its best endeavours to obtain the most favourable outcomes for the parties, to keep the LLP informed as to the progress of the Sales Strategy, to provide the LLP with details of all sales, offers and opportunities for exploitation of the Technology. It is also required to provide hard copies of all agreements, a report with details of pending agreements and a report of cancelled agreements to the LLP on a monthly basis (Clause 4.1.5) and provide the LLP with an account of all receipts and expenditure relating to the exploitation as it relates to a Drug (Clause 4.1.6) every six months.
101. Also, unless directed to the contrary, NPL must act in such manner as it considers "to be most beneficial to the LLP's commercial interests and reputation" (Clause 4.1.7).
102. Under Clause 6.1, NPL is to ensure that monies received from the exploitation of the Technology as it related to a given Drug would be paid into a separate account. The costs of implementing the Sales Strategy, and other costs of carrying on the business of marketing the Technology would, pursuant to Clause 6.2, be met from that account. Clause 6.3 provides that the profits were to be shared with 90% to NPL and 10% to the LLP.
103. Although the LLP and NPL entered into this JV Agreement, it was always more likely that NPL would exercise its rights under the Pre-emption rather than rely on the JV Agreement. This is clear from the financial projection in the IM (see above) which was based on the assumption that NPL would exercise its right of pre-emption and the absence of any similar projection in relation to the JV Agreement. Similarly the presentation slides for potential investors referred to NPL acquiring the technology from the LLP, "should the drugs pass testing." It was also consistent with the evidence of Mr Timol that if NPL thought it there was an opportunity to make money it was likely to exercise its pre-emption rights.
LLP Loan Facility
104. Under an agreement, dated 23 March 2015, between the LLP and NPL FC Limited ("NPL-FC"), referred to in the agreement as the "Lender", NPL-FC agreed, under Clause 2.1, to place at the LLP's disposal a loan facility of £5,920,000 (ie the 74% of the total funds referred to in the IM, see above).
105. Clause 3 provides that the purpose of the loan was to fund the R&D that the LLP had contracted for under the R&D Agreement. A covenant in Clause 5.1.1 also restricts the LLP to using the loan "only" to fund R&D which it has agreed to undertake under the Licence Agreement and/or which it has contracted for under the R&D Agreement.
106. There is a further covenant (at Clause 5.1.7) that the LLP will not pay any capital distribution out of the money provided by the loan or an amount up to the amount of the loan "subsisting from time to time" or (at Clause 5.1.8) to make any payment of capital or income to its members until the loan has been repaid in full.
107. Insofar as material, Clause 7 provides:
7. Limited Recourse
7.1 The Loan is only repayable out of Relevant Receipts.
7.2 The Lender's rights of claim as against the LLP and its members are limited from time to time to that amount which is the Relevant Receipts minus the sum of the amounts which have been repaid by the LLP to the Lender; and
7.3 To the extent that the amount which would otherwise be payable under the Loan exceeds the amount to which the Lender has a right of claim under paragraph 7.2 above, the Lender may also have recourse to any rights the LLP has under the Licence Agreement and/or the R&D Agreement [ie the Framework Agreement - see below] for repayment of the Loan.
7.4 In this facility letter "Relevant Receipts" means the Gross Relevant Receipts less Relevant Tax Liabilities.
7.5 "Gross Relevant Receipts" the amount from time to time is the sum total of:
7.5.1 the amount of any proceeds of exploitation of the rights acquired under the Licence Agreement; and/or
7.5.2 any amounts received by the LLP pursuant to the Licence Agreement; and/or
7.5.3 any amounts received by the LLP under the R&D Agreement.
7.6 "Relevant Tax Liabilities" means any liability to tax of any person which arises as a result of the LLP receiving the Gross Relevant Receipts.
108. It is clear from Clause 10, "Interest", that interest, which is chargeable at 7.5% per annum and compounded quarterly (Clause 10.1), is also on a limited recourse basis as Clause 10.2 provides that interest "shall be paid by the LLP at the same time as the principal amount outstanding under the Loan is repaid pursuant to clause 7."
Framework Agreement
109. As confirmed by Mr Timol in evidence, the LLP did not have the capacity, knowledge or workforce (or even the knowledge to hire staff) to undertake the R&D. It therefore entered into the Framework Agreement with NPL-Sub, a company incorporated and registered in the Republic of Cyprus, on 25 March 2015. This Agreement is the R&D Agreement as defined in the above Agreements.
110. It is accepted that, notwithstanding the Recital C in the Framework Agreement stating that NPL-Sub agreed "to perform the services, described in the Task Orders (as defined below) executed by the parties, in accordance with the terms and conditions of this Agreement", that NPL-Sub did not have the resources to undertake any R&D itself but would contract the work out to other parties.
111. Clause 1, the interpretation clause, contains many of the same definitions as in the other Agreements including "Confidential", "Completion of Research", the definition of Drugs 1 to 4 and the references to "Fully Funded", "Licence Agreement", "Research and Development" and "Services".
112. Under Clause 2 the LLP retained NPL-Sub to provide the R&D services in relation to the Drugs as described in Task Orders, a form of which was attached in Schedule 2 of the Agreement (for which see below). However, Clause 2.4 provides that the terms of a Task Order may be amended or modified by mutual written agreement of the NPL-Sub and the LLP.
113. NPL-Sub agreed during the term of each Task Order, under Clause 2.6, to maintain all materials and all other data it generated in the course of providing the relevant services including all electronic records and files (the "Work Product") in a secure area protected from fire, theft and destruction. NPL-Sub also agreed, pursuant to Clause 2.7, to complete the Services by the target date specified in the Task Order with time being of the essence. In the event of failure by NPL-Sub to perform the Services, or any portion of them, in accordance with the date or any later deadline set by the LLP, the LLP could terminate the Agreement and be immediately repaid the total amount of any credit.
114. At Clause 2.9 the parties acknowledged and agreed that by its nature R&D is:
"... speculative and nothing in this Agreement gives any representation that the Research and Development shall deliver any particular results or data."
115. Clause 3.1 required NPL-Sub to commence the Services within three months of receipt of the Initial Payment as agreed in the relevant Task Order.
116. Clause 5 provides for payments by the LLP to NPL-Sub for the provision of Services which was, as Dr Chowdhury confirmed in evidence, the only contribution the LLP could make.
117. Clause 5.1 provides:
"Each Task Order will provide a breakdown of the Research and Development services to be provided together with the fee agreed for this portion of the services to be provided. The cost of delivering the Research and Development (the "R&D Fee") shall be the sum of fees for the services specified in the Task Order and shall be specified in the Task Order."
118. Clause 5.4 makes provision for subsequent payments where the initial fee does not cover the full value of the R&D Fee.
119. Clause 6 provides for changes to the Services if it NPL-Sub reasonably determines that a continuation of the Services is unlikely to result in the profitable exploitation of the rights acquired by the LLP under the Licence Agreement.
120. Subsequent clauses provide for the duration and termination of the Agreement (Clause 7), Credit for unperformed Services (Clause 8), all intellectual property rights being for the benefit of NDM(Clause 9) and a right to assign the rights under the Agreement (Clause 11).
121. Under Clause 14.1 each party undertakes to the other that it shall keep (and procure that its respective directors, employees and contractors to whom it is disclosed) "secret and confidential" the Confidential Information of the other party. However, NPL-Sub is entitled, by Clause 14.3, to disclose Confidential Information to its members, employees or contractors to the extent that it is necessary for the purposes of this Agreement.
122. Schedule 1 to the Agreement provides for the Development Objectives, setting out in some detail (eg stage 2 which considers the need for clinical studies to be undertaken as laboratory models do not bear resemblance to human skin referring to the width and sharpness of a needle, depth of penetration and the likely skin trauma compared to the length of microneedles used the stages of the R&D to be undertaken).
123. Schedule 2 is headed "Task Order Template"
Task Orders
124. The parties to the Task Order are NPL-Sub and the LLP. Its recitals refer to the R&D Agreement, ie the Framework Agreement, which provides for NPL-Sub to provide Services upon payment by the LLP and that the Task Order specifies those Services to be Undertaken by NPL-Sub.
125. The Agreed Terms of the Task Order provide:
"1. In this Task Order, capitalised terms shall have the meanings given to them in the [Framework Agreement] except to the extent specified herein.
2. It is agreed that pursuant to the [Framework Agreement], the LLP shall pay NPL-Sub and Initial Payment of £2,015,385 (being an Instalment of the R&D Fee) upon entering this Task Order.
3. If the Initial Payment is not equal to the R&D Fee, LLP shall pay Instalments up [to] the value of the outstanding value of the R&D Fee in accordance with the [Framework Agreement] and applicable Supplemental Services Agreement.
4. [NPL-Sub] shall deliver the following research and development services in respect of the Initial Pair in order to meet the Development Objectives specified in Schedule 1 of the [Framework Agreement]."
126. Part A sets out the Research Stages. These are repeated in Part B, which is set out in the table below, the "Breakdown of the R&D Fee":
|
Element of the Services |
Cost |
|
Stage 1A: |
|
|
A Formulation Development and Optimisation |
£234,500 |
|
B Analytical Development
C Physical Characterisation |
£234,500 |
|
D Device Development and optimisation |
£201,000 |
|
Stage 1B: Scale-up studies:
· Batch size and process parameter
· Quality by design studies
· In-process control parameter determination
· Quality control methods
· Product release specification
· Packaging compatibility and stability studies |
£165,000 |
|
Stage 1C: Pre-Clinical studies in animal models
· Ethics approvals
· Study design
· Formulation preparation and release
· Device preparation and release
· Product application optimisation
· Bio-analytical method development and validation |
£165,000 |
|
Stage 2A: Clinical Trial Batch Manufacture: Device
· Equipment design and manufacture
· Process design and equipment procurement
· Validation of equipment and process
Stage 2B: Clinical Trial Batch Manufacture: Drug Formulation
· Scale up process development
· In-process testing
· Quality assurance testing and release
· Qualified-person - product release
· Statistical process control |
£900,000
|
|
Stage 2C: First in man studies
· Clinical protocol design
· Ethics approval
· first in man study - 12-20 volunteers
· bio-analytics
· clinical report |
£150,000 |
|
Stage 2D: Long term stability studies
· ICH guidelines on stability testing - to 2 years
· Stability test method development
· Statistical evaluation of stability samples |
£300,000 |
|
Stage 2E: Bridging Clinical Studies in man
· Clinical Studies in man
· Pilot pharmacokinetic studies - 10-12 healthy volunteers
· Volunteer studies - up to 225 patient study
· Ethics approvals
· Clinical study protocol
· Bio-analytical method development
· Statistical analysis |
£1,200,000 |
|
R&D FEE |
£4,000,000 (sic) |
127. The total cost of the services, the R&D Fee, as set out above is actually £3,550,000 not £4,000,000. Mr Mullan referred to this as an "arithmetical error", something that was replicated in the initial Task Order, dated 26 March 2015. Mr Afzal, in contrast, described it as "an error of substance, which then goes to commerciality".
128. However, the total cost, as set out in a summary included in a OneE September 2015 investor update that set out the stages and costs of "the scope of the work expected to be required", did correctly amount to £4 million.
129. This is also the case with regard to an updated Task Order, dated 26 April 2016, giving further details of the various "stages" which had not been included in the earlier Task Order, as described in the above table. These include references to "Analytical method validation" at Stage 1A, "Pharmacokinetic studies - 10-12 healthy volunteers" at Stage 2D and "dossier compilation, chemistry manufacture and controls, clinical data, toxicology data" at Stage 2E.
130. The 26 April 2016 Task Order provides:
|
Element of the Services |
Cost |
|
Stage 1A: The following four areas will run in parallel |
|
|
Formulation Development and Optimisation |
|
|
· Drug Compatibility studies (including preclinical development) |
£35,175 |
|
· Excipient compatibility studies (including preclinical development) |
£35,175 |
|
· Stress Studies |
£35,175 |
|
· Temperature and humidity studies in accordance with ICH guidelines |
£35,175 |
|
· Chemical characterisation |
£93,800 |
|
|
|
|
|
£234,500 |
|
Analytical Development |
|
|
· Excipient characterisation |
£23,450 |
|
· Drug characterisation |
|
|
· Impurity detection and characterisation |
£58,625 |
|
· Solvent detection |
£11,725 |
|
· Residual substance detection |
£11,725 |
|
· Analytical method validation |
£70,350 |
|
|
£175,875 |
|
Physical Characterisation |
|
|
· Solubility |
£58,626 |
|
· Dispersion |
|
· Homogeneity of active pharmaceutical |
|
· Ingredients and crystal seeding/precipitation |
|
|
£58,625 |
|
Device Development and Optimisation |
|
|
· Material selection |
£10,050 |
|
· Material specification determination |
£10,050 |
|
· Material compatibility |
£20,100 |
|
· Device Housing |
£30,150 |
|
· Needle assembly |
£20,100 |
|
· Liquid flow property determination |
£20,100 |
|
· Skin application and ease of insertion |
£20,100 |
|
· Needle insertion forces |
£30,150 |
|
· Skin-needle-drug release inter-relationship |
£20,100 |
|
· Device scale-up |
£20,100 |
|
|
£201,000 |
|
|
|
|
Stage 1B: Scale-up Studies (including Quality by Design Studies) |
|
|
· Batch size and process parameter |
£41,250 |
|
· In-process control parameter determination |
£41,250 |
|
· Quality control methods |
£41,250 |
|
· Product release specification |
£24,750 |
|
· Packaging compatibility studies and stability studies |
£16,500 |
|
|
£165,000 |
|
|
|
|
|
|
|
Stage 1C: Pre-clinical Studies in Animal Models |
|
|
· Ethics approvals |
£8,250 |
|
· Study design |
£8,250 |
|
· Formulation preparation and release |
£41,250 |
|
· Device preparation and release |
£41,250 |
|
· Product application optimisation |
£33,000 |
|
· Bio-analytical method development and validation |
£33,000 |
|
|
£165,000 |
|
|
|
|
Stage 2A: Clinical Batch Manufacture (including Statistical Process Control) |
|
|
· Equipment design and manufacture |
£135,000 |
|
· Process design and equipment procurement |
£90,000 |
|
· Validation of equipment and process |
£135,000 |
|
· Scale-up process development |
£270,000 |
|
· In-process testing |
£135,000 |
|
· Quality assurance testing and release |
£90,000 |
|
· Qualified person - product release |
£45,000 |
|
|
£900,000 |
|
|
|
|
Stage 2B: First in Man Studies |
|
|
· Pilot pharmacokinetics studies |
£300,000 |
|
|
£300,000 |
|
|
|
|
Stage 2C: Long Term Stability Studies |
|
|
· ICH guidelines on stability testing - to 2 years |
£165,000 |
|
· Stability test method development |
£60,000 |
|
· Stability test method validation |
£60,000 |
|
· Statistical evaluation of stability samples |
£15,000 |
|
|
£300,000 |
|
|
|
|
Stage 2D: Clinical Studies in Man |
|
|
· Pharmacokinetic studies - 10-12 healthy volunteers |
£187,500 |
|
· Volunteer studies - up to 225 patient study to cover skin irritation, sensitisation, pharmacokinetics and where appropriate pharmacodynamics and skin toxicology |
£875,000 |
|
· Ethics approvals |
£62,500 |
|
· Clinical study protocol |
£31,250 |
|
· Bio-analytical method development |
£62,500 |
|
· Statistical analysis |
£31,250 |
|
|
£1,250,000 |
|
|
|
|
Stage 2E: Data Evaluation and Analysis |
|
|
· Dossier compilation |
£62,500 |
|
· Chemistry manufacture and controls |
£87,500 |
|
· Clinical data |
£87,500 |
|
· Toxicology data |
£12,500 |
|
|
£250,000 |
|
|
|
|
R&D Fee |
£4,000,000 |
131. In relation to the completion of the elements of services as stated in the Task Order, a 2023 progress update confirms that the R&D on lidocaine had been completed to stage 2B and that on risperidone to stage 1C.
Pricing
132. The prices, as set out above, constituting the R&D fee had been subject to discussion to ensure that what was being charged was, as Mr Mullan put it in his submissions, "reasonable and sensible." This is apparent from an email chain from which it would appear that quotations were sought from three third party R&D providers.
133. The first email in the chain, dated 4 March 2015, is from Kin Chu ATT, a tax consultant with OneE, to Dr Chowdhury in which he wrote:
"Hi Faz,
As mentioned today, we did get a number of responses back, attached.
Parexel have reverted stating that their London base does not have the capacity. I went back asking for a ball park estimate for this being in SA and their latest response is querying the actual testing to be done for an injection based device.
Likewise the response from MedPharm.
The third company is yet to come back to me.
Is there a high-level response you could provide to these emails or is this going to require much more substantial information being disclosed (which I assume we would want to avoid)."
134. In a further email, of 19 March 2015, Kin Chu notified Dr Chowdhury that Parexel had "suddenly reverted back with a quote" and asked Dr Chowdhury whether he was able to "shed some light on the quotes provided" and how these compared to the projected costs. Dr Chowdhury responded later that day that:
"Parexel have provided a very competitive quote for Europe.
The costs compare well ‐ and this is a good document to have on file.
We've said about £1.2m - and they have quoted iro £1.3-£1.4m for the clinical bridging study."
135. In evidence, Dr Chowdhury explained that although Kin Chu was a tax adviser he was dealing with the administrative aspects to assist NPL by gathering quotes. He also said that he had referred to the quotation as being a "good document" as it showed OneE and the LLPs that NPL was competitive and evidenced that its prices were "in tune with what the industry norms were in terms of the charges that are levied."
136. A further email exchange took place between Kin Chu and Timothy Johnson CTA, a director of OneE, in April 2016 (which postdates the Framework Agreement). It concerned an attachment containing a breakdown of costs set against third party quotations. An email from Kin Chu to Timothy Johnson, dated 8 April 2016 explained:
"The attached [ie the breakdown of costs vs third party quotes] is what we have in terms of quotes. The only one we got directly was the Parexel quote, all the rest were details supplied by Faz [Dr Chowdhury]. The two other companies we approach said no or ignored us."
137. On 27 July 2016 Timothy Johnson sent the following email to Kin Chu:
"Hi Kin,
I know we have approached and got quotes from other firms for doing the work for R&D. I'm not sure how may we have and the scope of the same. Can we please meet to review the same as it is quite an important factor in light of the Brain Disorders case."
Payments (under Framework Agreement)
138. The LLP made the following payments to NPL-Sub in accordance with Clause 5 of the Framework Agreement (which are the subject of this appeal):
(1) £2,015,385 in respect of the Task Order dated 26 March 2015;
(2) £1,925,000 in respect of the Task Order dated 30 April 2015;
(3) £1,258,077 in respect of the Task Order dated 28 May 2015;
(4) £2,195,769 in respect of the Task Order dated 30 June 2015;
(5) £490,384 in respect of the Task Order dated 28 August 2015; and
(6) £115,384 in respect of the Task Order dated 22 September 2015.
Secondary R&D Agreements
139. The Framework Agreement is silent on whether NPL-Sub would itself sub-contract the R&D.
140. However, an agreement, dated 26 March 2015, between NPL-Sub and NPL, sets out the work to be undertaken for which NPL-Sub "shall contract for research to be undertaken" and NPL agrees to perform the "Services" (which in essence reflect the services set out at Stage 1 in first Task Order above) in accordance with that Agreement. Further agreements between NPL-Sub and NPL were entered into on materially similar terms on 6 September 2016 and 10 January 2018 which covered later stages of the work to be completed.
141. Clause 2 provides that, subject to Clause 3, NPL agrees to commence the Services in relation to Drug 1 (lidocaine) and Drug 2 (risperidone) within three months. Clause 3 provides for payment under the Agreements.
142. Under clause 7.2 it was made clear that:
"All Intellectual Property Rights arising out of this Agreement (including those before the execution of this Agreement) shall be for the benefit of NDM and [NPL] shall at the direction of (NPL-Sub) assign such rights to NDM."
143. Although the total consideration due under these agreements was £3.2 million, payments actually totalled £2.6 million. These were recorded in a number of Supplemental Services Agreements: £292,322 on 26/27 March 2015; £282,162 on 12 May 2015; £185,706 on 2 June 2015; £324,119 on 2 July 2015; £72,386 on 1 September 2015; £17,032 on 2 October 2015; £826,273 on 12 December 2016; £300,000 on 14 December 2016; and £300,000 on 12 January 2018. The last three of these payments (totalling £1,426,273) were made by way of an assignment of creditors rights against NPL-FC from the NPL-Sub to NPL.
Engagement letters
144. In a series of engagement letters OneE charged NPL-Sub a services fee for introducing the LLP to NPL-Sub and engaging it to provide R&D. Charges (exclusive of VAT) under these letters (totalling £906,093) were £231,648 on 26 March 2015; £218,308 on 12 May 2015; £141,364 on 2 June 2015; £246,751 on 2 July 2015; £55,084 on 1 September 2015; and £12,938 on 2 October 2015.
NPL-FC Loan Facility
145. NPL-Sub, as lender, made a made a series of loans (totalling £5,919,970) to NPL FC– see above), as borrower as follows:
(1) £1,491,385 on 26 March 2015;
(2) £1,424,500 on 12 May 2015;
(3) £930,977 on 2 June 2015;
(4) £1,624,869 on 2 July 2015;
(5) £362,855 on 1 September 2015; and
(6) £85,384 on 2 October 2015.
146. As noted above, £1,426,273 of these amounts are now owed to NPL having been assigned in consideration of the provision of R&D services under the Secondary R&D Agreements.
147. By a loan facility amendment deed between the NPL-FC and the NPL-Sub, dated 13 December 2022, it was agreed that interest ceased to run from 1 January 2018 and that instead the NPL-Sub would be entitled to the NPL-FC's profit entitlement under the LLP Loan Facility as amended.
Consultancy Agreement
148. On 12 November 2015, the LLP entered into a Consultancy Agreement with OneE under which OneE was appointed, amongst other things, to monitor the LLP's business and deal with its administrative and managerial duties. Clause 3.2.1 of the Agreement provided that the Consultancy Fee shall be:
"25% of any profits of the [LLP] once the Subscribing Members have received Distributions in an aggregate equal to their Application Amount (as stated in that Subscribing Member's Application Form as the "Application Amount")."
149. The consultancy services to be provided to the LLP are set out in Schedule 1 to the Consultancy Agreement and are divided into "Initial" and "Ongoing" services.
150. The Initial Consultancy Services included the procuring of such legal, accounting and other professional services as OneE deemed appropriate in addition to assisting the LLP in the preparation of budgets and forecasts and all aspects of due diligence recommended by OneE in respect of the LLP's business.
151. The Ongoing Consultancy Services included monitoring the ongoing partnership business, the provision of advice, preparation of accounts, budgets and forecasts and procuring insurance policies as necessary. It also included assisting the LLP with its preparation for reports and other communications to its members in addition to providing assistance in relation to the LLP's other administrative and managerial duties, roles and functions.
152. This Agreement was novated to OneE Trustee Services Limited on 5 June 2020
Deed of Variation
153. On 25 March 2020, NPL,NDN, OneE and the 11 LLPs, including the LLP, engaged in R&D relating to the transdermal patch entered into a Deed of Variation (the "Deed of Variation"). This amended the JV Agreement and the Pre-Emption Agreement to provide that an additional amount of up to £2 million was payable from NPL's profit share out of the exploitation of any of the research carried out by the 11 LLP's. This amount (totalling £22 million) was payable to each LLP.
154. HMRC's view is that because it was entered into in 2020, some five or six years after the structure was entered into, the Deed of Variation has little bearing on the position in the years of appeal.
Subsequent Events
155. Dr Chowdhury explained that unforeseen events that occurred after 2015, particularly Brexit and the coronavirus pandemic, had an adverse effect on the development of various molecules. These led to changing markets and a downturn in investor sentiment in high risk projects in addition to the effects of the closure of laboratories during the various lockdowns.
156. Email correspondence, from 2016 onwards, refers to the provision of updates for the members of the LLP on the progress of the R&D and the likely completion dates for each of the molecules. For example, an email dated 4 April 2017 from Dr Chowdhury to Maryam Bawa of OneE, responding to an email of 3 April 2017 asking about progress of the stages in relation to risperidone which had been expected to have been completed by the end of March 2016, stated:
"Risperidone is a very complex formulation of microparticles with very specific drug release profile. We have thus far completed optimisation of the formation of the particles and this is being further optimised to achieve the requisite release properties, but due to the nature of the drug release measurements (over 2 weeks per study) the stages below have not as yet been completed
We anticipate this project will require a further 3 months to complete this stage."
157. There were also similar emails in respect of lidocaine. It is clear from these that progress had not been as swift as anticipated as can be seen from a series of emails from the period around December 2018/January 2019, between OneE (Timothy Johnson and Mr Timol) and Dr Chowdhury discussing how best to update the LLP's members. One such email, dated 14 January 2019, from Dr Chowdhury includes the following:
"To date we have not as yet had to drop any drugs, although some have proven to be extremely challenging and requiring further development. Our reasons for not dropping even the most complex of drugs, thus far, is that firstly we are confident we can resolve the existing issues, and secondly the higher the challenge the greater the barrier to entry and therefore the higher asset value, thus in everyone's interest.
Given the structure of the LLP's I am not sure how we go about addressing the point of potentially allowing everyone to benefit from 'all' molecules to some extend, so perhaps you can share you thoughts on this, as it is obviously not going to be possible through the potential EIS scheme."
158. It was the discussion in the second paragraph quoted above which ultimately led to the Deed of Variation being agreed in March 2020 as a "goodwill gesture" to investors who had been notified of this proposal in their February 2019 investment update.
159. It is clear from the emails received that the investors were not completely satisfied about the way in which their investment had been managed by the LLP. One such email, dated 7 February 2020, from an investor stated:
"... that given the track record of never producing anything either on time or of any meaning over the last six years, I would be very surprised if you provided anything either on time or [of] any substance."
Money Movements
160. It is convenient at this stage, having referred to the explanation in the IM of how the LLP was to be funded (ie by capital contributions of the members of the LLP (26%) and third party loans (74%)) and its financial commitments under the various agreements, to summarise the movements of money through the various entities.
161. As illustrated in the diagram below (which was taken from an appendix to HMRC's skeleton argument) with the figures in bold being the percentage of the funds utilised:
(1) The LLP paid £8 million (100%) to NPL-Sub to carry out R&D. Of that £8 million, £2,080,000 (26%) came from cash contributions from members and £5,920,000 (74%) from a loan to the LLP from NPL-FC on a limited-recourse basis, with NPL having borrowed those monies from OneE.
(2) NPL-Sub lent £5,920,000 (74%) to NPL-FC which used those sums to repay its loans from OneE.
(3) NPL-Sub used the £2,080,000 (26%) to:
(a) pay fees of £906,093 (11%) to OneE (as stipulated in the Letters of Engagement dated 26 March 2015, 12 May 2015, 2 June 2015, 2 July 2015 and 1 September 2015;
(b) pay £1,173,727 (15%) to NPL to carry out R&D; and
(c) pay £180 in "Funds Transfer Fees" (not included in the diagram).
(4) In addition to the £1,173,727, NPL was also assigned debts by NPL-Sub totalling £1,426,273 (ie £826,273 plus £300,000 plus £300,000) making a total of £2,600,000. NPL-Sub has never paid the remaining £600,000 outstanding under the third NPL R&D Agreement.
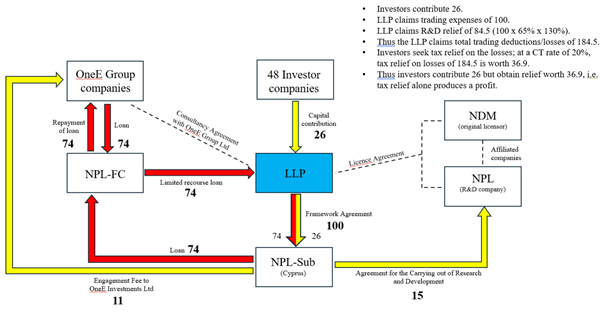
162. It should also be noted that, although not shown in the above diagram, there was, as Mr Mullan pointed out, an assignment of a right to repayment of the loan by NPL-Sub to NPL which totalled £1,426,273 (18%). Mr Mullan also contends that there was delivery of £2.9 million (36%) R&D to the LLP by NPL. However, we agree with HMRC which does not accept that there was "delivery" of R&D as it is clear from the above Agreements the product of any R&D belonged to NPL.
Issues
163. It is common ground that the following issues arise:
(1) Whether the LLP was trading;
(2) Whether the LLP was carrying on a trade or business "with a view to profit" for the purposes of s 1273 CTA 2009;
(3) Whether any deduction in respect of the R&D costs is precluded by s 54 CTA 2009 on the basis that the expenditure was not incurred "wholly and exclusively" for the purposes of the LLP's trade;
(4) Whether R&D relief is available; and
(5) Whether the Framework Agreement reflects the true agreement of the parties.
164. Although not necessarily in the order in which they were addressed in submissions, we consider it more appropriate to consider the issues in the order above, taking the trading issue first, given the effect that the decision on that issue will have on the others.
165. As the Court of Appeal observed in Ingenious Games LLP and others v HMRC [2022] 2 All ER 338 ("Ingenious") at [113], the "view to a profit" issue only arises if the LLP is held to be carrying on a trade. Also, if there is no trade it is not possible for the LLP to have incurred expenditure "wholly and exclusively" for the purposes of a trade. Similarly, an entitlement to qualify for R&D relief can only arise where the company "carries on a trade" (see s 1044(4) CTA 2009).
166. Therefore, if we are not with the LLP on the trading issue its appeal cannot succeed.
Trading
167. There is very little legislative guidance on the meaning of "trade". Both s 1119 of the Corporation Taxes Act 2010 ("CTA 2010") and s 989 of the Income Tax Act 2007 provide that:
"trade" includes any venture in the nature of trade
168. As Sir Terence Atherton C (as he then was) observed, giving the judgement of the Court of Appeal, in Eclipse Film Partners (No 35) LLP v HMRC [2015] STC 1429 ("Eclipse CA"):
"111. ... It is necessary to stand back and look at the whole picture and, having particular regard to what the taxpayer actually did, ask whether it constituted a trade.
112. The Income Tax Acts have never defined trade or trading further than to provide that (in the words of TA 1988, s 832(1) which was applicable to the relevant tax year) trade includes every trade, manufacture, adventure or concern in the nature of trade. As an ordinary word in the English language 'trade' has or has had a variety of meanings or shades of meaning. Its meaning in tax legislation is a matter of law. Whether or not a particular activity is a trade, within the meaning of the tax legislation, depends on the evaluation of the activity by the tribunal of fact. These propositions can be broken down into the following components. It is a matter of law whether some particular factual characteristic is capable of being an indication of trading activity. It is a matter of law whether a particular activity is capable of constituting a trade. Whether or not the particular activity in question constitutes a trade depends upon an evaluation of all the facts relating to it against the background of the applicable legal principles. To that extent the conclusion is one of fact, or, more accurately, it is an inference of fact from the primary facts found by the fact-finding tribunal."
169. In Samarkand Film Partnership No 3 and others v HMRC [2017] STC 926 ("Samarkand") Henderson LJ noted, at [59], that:
"... At the most basic level, it is now clear from Eclipse, if it was not clear before, that the question whether what the taxpayer actually did constitutes a trade has to be answered by standing back and looking at the whole picture: see [111]. Although it is a matter of law whether a particular activity is capable of constituting a trade, whether or not it does so in any given case 'depends upon an evaluation of all the facts relating to it against the background of the applicable legal principles': see [112]. It follows that it can never be appropriate to extract certain elements from the overall picture and treat them, viewed in isolation, as determinative of the issue. ..."
170. Mr Afzal contends that it is clear from the documents, eg Clause 3.5 of the Licence Agreement and similar clauses in the other Agreements (that any research intellectual property and/or research results shall vest in and be owned absolutely by NDM), that in reality all the LLP was doing was "putting up some money" with the possibility that it might get a return without actually doing anything. This is because the R&D was being undertaken by someone else with the results of that R&D also belonging to someone else. This he says is a "classic investment" activity rather than a trading activity.
171. Mr Mullan contends that HMRC's approach does not take account of the overall picture. Rather, it takes facts in isolation as determinative of the issue, and does not take account of what occurred after March 2015. Although he accepts that the funding arrangements does "create an element of wrinkle", Mr Mullan submits that, based on the documents and the evidence of the witnesses it is clear that the LLP was trading as it acquired rights with a view to developing them and off-selling them at a considerable profit.
172. He compares the actions of the LLP to the acquisition of a cargo vessel, its conversion into a steam drifter and subsequent sale by a ship repairer, blacksmith and fish salesman in Inland Revenue Commissioners v Livingston and Others 11 TC 538, which was held by the Court of Session to be a trading transaction.
173. In that case the Lord President (Clyde) said, at 543,
"... It may be that, in commercial practice relative to ships, this kind of business is not usually followed separately from the general business of shipbuilders and ship-repairers. But, even so, I think it is none the less "in the nature of trade." The profit made by the venture arose, not from the mere appreciation of the capital value of an isolated purchase for resale, but from the expenditure on the subject purchased of money laid out upon it for the purpose of making it marketable at a profit. That seems to me of the very essence of trade."
174. Mr Afzal, with whom we agree, distinguishes Livingston on the facts which he says are fundamentally different and does not have the convoluted structure that exists in the present case. Mr Afzal also referred to the observation of Lord Blackburn in that case (at 546-547) who would have had "some difficulty" in agreeing that there was a trade if the alteration and repair of the vessel had been "carried out by a stranger" rather than taxpayer's themselves.
175. In addition to Livingston, Mr Mullan relies on the Upper Tribunal decision in Vaccine Research Ltd Partnership and another v HMRC [2015] STC 179 ("Vaccine Research") which, like the present case, concerned a claim for R&D relief in which the Upper Tribunal (Sales J, as he then was, and Judge Ghosh QC) found, at [58] "no good grounds for interfering with the FTT's conclusion" that the Partnership in that case was trading.
176. At [57] the Upper Tribunal observed that:
"The research agreement, the sub-research contract (and the other documentation which implemented the 'Scheme') were all 'commercial' in the sense that they had as part of their objective research and development of a vaccine or vaccines which it was hoped would yield royalties and the royalties which might become due under the research sub-contract were clearly a 'reward' from PepTcell Ltd (which was, at the same time, the 'Appointed Sub-Contractor' under the research agreement, subject to an obligation to develop successful vaccines, and the Partnership's 'customer' under that agreement, who paid for the right to exploit the patents and inventions held by the Partnership by agreeing to pay the royalties). The royalties were not income simply to be enjoyed by the Partnership qua owner of an income-producing asset. The Partnership had to fund the activities of PepTcell Ltd, via its arrangements to fund Numology Ltd, to carry out research and develop the vaccines into something which might ultimately prove to be marketable so as to generate an income. Nor did the royalties represent simply a form of capital appreciation, for the same reasons. The Partnership had to arrange for research and development activities to be funded and carried out in order to have any hope of a return on its assets. Accordingly, in the circumstances of this case, the Partnership's activity could not be regarded as merely making an investment in an income-producing or capital-appreciating asset. The FTT was plainly entitled to consider the Partnership's activity to be business or commercial activity, which did not have the character of investment activity; similarly, the FTT was plainly entitled to consider that the Partnership's activity was trading activity."
177. However, in Brain Disorders Research Ltd Partnership v HMRC [2018] STC 2382, which concerned similar arrangements to those in Vaccine Research, the Court of Appeal upheld the conclusions of the First-tier and Upper Tribunals that there was not trading activity.
178. Having been referred to and considered, in addition to those cited above, the various authorities on trading, including eg Ransom v Higgs [1974] STC 539, the "badges of trade" in Marson v Morton [1986] STC 463, Ensign Tankers (Leasing) Ltd v Stokes [1992] 1 AC 655, and what can be described as the "Film Scheme" cases etc, it is necessary to stand back and look at the whole picture with the focus on the LLP and what it actually did, and not attempt to draw analogies with the facts of other cases.
179. We have found assistance in this approach from the badges of trading to which Sir Nicolas Browne-Wilkinson VC (as he then was) referred in Marson v Morton (at 470-471). We have taken these into account together with the submissions on them from both Mr Mullan and Mr Afzal while keeping in mind, as Sir Nicholas Browne-Wilkinson observed, at 471, that they:
"... are not a comprehensive list and no single item is in any way decisive. I believe that in order to reach a proper factual assessment in each case it is necessary to stand back, having looked at those matters, and look at the whole picture and ask the question-and for this purpose it is no bad thing to go back to the words of the statute - was this an adventure in this an adventure in the nature of trade? In some cases perhaps more homely language might be appropriate by asking the question, was the taxpayer investing the money or was he doing a deal?"
180. The first of those badges, is to consider whether "the transaction in question was a one-off transaction". Of this badge, Sir Nicolas Browne-Wilkinson said:
"Although a one-off transaction is in law capable of being an adventure in the nature of trade, obviously the lack of repetition is a pointer which indicates there might not here be trade but something else."
181. Mr Mullan accepts that there is a one-off transaction in the sense that the LLP is engaging in relation to just two molecules.
182. However, he says, that it is necessary to consider this in the context of the LLP's relationship with NPL which is clearly trading in relation to many different molecules. He contends that if that is the case, the question should not depend on the number of molecules, especially when considered in the wider context of what was happening. However, we agree with Mr Afzal who contends that there was no repetition. There was one Licence Agreement and one Framework Agreement. Also, as is clear from the authorities, the question is solely in respect of the LLP and not the wider context in relation to NPL.
183. The second badge is whether the transaction in question is in some way related to the trade which the taxpayer otherwise carries on. The example given by Sir Nicolas Browne-Wilkinson was that a:
"one-off purchase of silver cutlery by a general dealer is much more likely to be a trade transaction than such a purchase by a retired colonel."
184. Again, Mr Mullan sought to link the LLP with NPL's existing trade, the development of transdermal patches. However, that cannot be right as this badge must relate to the trade of the person making the transaction, in this case the LLP not NPL. Here the LLP had no pre-existing trade and therefore the transaction with which this appeal is concerned has no trade with which it can be connected.
185. The third badge concerns the nature of the subject matter. Sir Nicolas Browne-Wilkinson considered that this, "may be a valuable pointer." In the present case, there was some dispute as to the nature of the subject matter. Mr Afzal described it as "essentially intellectual property" whereas Mr Mullan said it was the right to exploit the Technology, ie the transdermal patch technology, that had been acquired.
186. Notwithstanding reference in recital (C) to the Licence Agreement stating NDM's intention to grant the LLP an "exclusive licence to exploit the use of the Technology", under Clause 2.1 of that Agreement (see above), what NDM actually granted the LLP was an exclusive, worldwide, royalty-free licence to "exercise all and any of NDM's rights and privileges in and to the Technology" insofar as it related to each of the Drugs.
187. We agree with Mr Afzal that this is not a right to exploit, but the acquisition of intellectual property which is more typical of an investment than trading, particularly as, in this case, the most likely way in which the LLP was going to make a profit was by disposing (rather than exploiting) this right pursuant to the Pre-emption deed.
188. Turning to the fourth badge, the way in which the transaction was carried through and whether it was carried through in a way typical of the trade in a commodity of that nature?
189. Mr Mullan accepts that the LLP was not trading in a commodity but contends that the situation is analogous to Livingston in terms of a business model, buying an asset, working on it to increase its value before selling it off. He also says that the LLP was doing nothing different from NPL and that trade can be carried on in different ways. Other than agreeing that trade can be carried on in different ways, we reject his submission on this badge.
190. As noted above, Livingston can be distinguished from the present case and, as is clear from the evidence of Dr Chowdhury (see above), the activities of NPL and the LLP are completely different. The LLP was, on Mr Mullan's argument exploiting the Technology and did not, as Mr Timol confirmed in evidence, have the capacity to carry out R&D itself or even the knowledge to hire staff to do so whereas NPL, as described in the IM and by Dr Chowdhury in evidence, was able to undertake R&D "in house" from the basic concept through to the end product at its laboratories.
191. The fifth badge, the source of finance. Sir Nicolas Browne-Wilkinson observed that:
"...If the money was borrowed that is some pointer towards an intention to buy the item with a view to its resale in the short term; a fair pointer towards trade."
192. In the present case there was, as Mr Mullan submits, clearly borrowing. However, Mr Afzal, with whom we agree, accepts, as he must, that there was borrowing but contends that as this was on a limited recourse basis (see eg clause 7 of the LLP Loan Facility) there was no imperative to repay the loan and, as such, the pointer towards trade does not exist.
193. In relation to whether there was any work done on an item or if it was resold as it stood, the sixth badge, Mr Afzal contends that nothing was actually done by the LLP and, as is clear from eg Clause 3.5 of the Licence Agreement (see above), none of the benefit of the R&D accrued to it. As such, he says this badge was not present.
194. However, Mr Mullan submits that the whole purpose of the arrangements in the present case was that R&D would be undertaken on or by reference to the LLP's rights under the agreements which would make those rights more valuable and therefore the LLP would benefit from any R&D work. Mr Mullan also points to the R&D relief legislative provisions which clearly envisage the subcontracting out of R&D work and which can only apply where there is trading.
195. While there is no doubt that is correct, we agree with Mr Afzal who submits that it does not follow that the LLP is trading just because the R&D has been subcontracted.
196. It is common ground that the seventh badge, whether the item broken down into saleable lots, does not apply in the present case
197. The eighth badge concerns the intentions of the purchaser as to resale at the time of purchase. Sir Nicolas Browne-Wilkinson noted that "an intention to resell in the short term rather than the long term is some indication against concluding that the transaction was by way of investment rather than by way of a deal." However, he also observed that so far as he could see, "this is in no sense decisive by itself."
198. Mr Mullan referred to the original intention in the present case that the money would flow back into the system in the short-term but, because of circumstances including Covid, Brexit and a changing market, the length of the project increased. Mr Afzal, however, contends that even the initial time frame of around three years, as presented to potential investors at the seminars and roadshows, was fundamentally long term and is consistent with investment. We agree.
199. Mr Afzal contends the ninth and final badge, whether the item was purchased for enjoyment for the purchaser (Sir Nicolas Browne-Wilkinson's example was a painting) or to produce income pending resale, was neutral in the present case. However, Mr Mullan disagrees and submits that as there is neither income produced nor pride of purchase spending, if anything, as Sir Nicolas Browne-Wilkinson said, it "is a strong pointer in favour of it being a trade rather than an investment."
200. Standing back and taking all of the circumstances and submissions of the parties into account (even if not specifically mentioned), particularly in the light of our observations in relation to the badges of trading (see above), with our focus on the LLP (not NPL) we have come to the conclusion that, on balance, the activities of the LLP are more consistent with an investment than being a venture in the nature of a trade.
201. As this conclusion is sufficient to dispose of the appeal it is not strictly necessary for us to address the remaining issues. However, as these issues were argued before us, and in case of any further appeal, we have set out our conclusions with brief explanations rather than deal with them as comprehensively as would have been the case had we come to a different conclusion in relation to the Trading Issue.
Remaining Issues
202. Had we decided that the LLP was trading, which we did not, we would have concluded that:
(1) it was doing so "with a view to profit" for the purposes of s 1273 CTA 2009;
(2) the expenditure on R&D was not incurred "wholly and exclusively" for the purposes of the LLP's trade and, pursuant to s 54 CTA 2009, is therefore not deductible;
(3) R&D relief was available; and
(4) the Framework Agreement reflects the true agreement of the parties.
View to a Profit
203. The "view to a profit issue was considered in some detail in Ingenious (see at [113] - [168]), in which it was agreed the words, 'with a view to a profit', "import a wholly subjective test" and that "it must be the actual subjective intention or purpose of the putative partners to make profits from carrying on their trade (see at [119]).
204. It was also confirmed, at [122] in Ingenious, that there was "no objective element to the requirement for a view to profit". At [123] in Ingenious the Court of Appeal observed that it was "uncontroversial" that 'profit' had an objective meaning, that there was no maximum period during which the partners must intend to make a profit and that 'profit' has the basic meaning of an excess of income over costs.
205. The Court of Appeal in Ingenious also cited, with approval, the Canadian case of Blackman v R 2001 SCC 10 in which it was noted that a partnership may incur initial losses during the start up phase of its enterprise but that this:
"...does not mean that the relationship is not one of partnership, so long as the enterprise is carried on with a view to profit in the future."
206. In our view, although there was no business plan as such, the documentation, in particular the IM, the Roots market research (notwithstanding its caveats), the Pre-emption Agreement and JV Agreement, points to the LLP, had it been trading, doing so with a view to a profit.
Wholly and Exclusively
207. In Vodafone Cellular v Shaw [1997] STC 734 at 742, Millet LJ (as he then was), having considered Mallalieu v Drummond (Inspector of Taxes) [1983] STC 665 and MacKinlay (Inspector of Taxes) v Arthur Young McClelland Moores & Co [1989] STC 898, which he described as the "leading modern cases on the application of the exclusively test", derived the following principles:
"(1) The words for the purposes of the trade mean to serve the purposes of the trade. They do not mean for the purposes of the taxpayer but for the purposes of the trade, which is a different concept. A fortiori they do not mean for the benefit of the taxpayer.
(2) To ascertain whether the payment was made for the purposes of the taxpayer's trade it is necessary to discover his object in making the payment. Save in obvious cases which speak for themselves, this involves an inquiry into the taxpayer's subjective intentions at the time of the payment.
(3) The object of the taxpayer in making the payment must be distinguished from the effect of the payment. A payment may be made exclusively for the purposes of the trade even though it also secures a private benefit. This will be the case if the securing of the private benefit was not the object of the payment but merely a consequential and incidental effect of the payment.
(4) Although the taxpayer's subjective intentions are determinative, these are not limited to the conscious motives which were in his mind at the time of the payment. Some consequences are so inevitably and inextricably involved in the payment that unless merely incidental they must be taken to be a purpose for which the payment was made."
208. Millet LJ added a further proposition to these, that the "primary inquiry" is to ascertain what was the particular object of the taxpayer in making the payment and once that is ascertained, "its characterisation as a trade or private purpose" is a matter for the Tribunal.
209. It is therefore necessary to ascertain the purpose of the LLP incurring the £2.9 million. As Nugee J (as he then was) observed in Acornwood LLP v HMRC [2016] STC 2317 at [54]:
"... what is significant is the purpose of the payer, not what the recipient does with the money... But if the payer knows that the payee is going to use the money in a particular way, and intends that the payee should do so—indeed has been responsible for devising the transaction in such a way as to make it essential that the payee does use the money in that way—then it is wholly unrealistic to say that the payer does not intend the money to be used for that purpose. And if that is what the payer intends, it is very difficult to see that the payer can have had any other object in making the payment."
210. Mr Mullan says the money was paid by the LLP for R&D and, as such, it is not precluded from deduction under s 54 CTA 2009. However, given Mr Timol's comment, albeit one he retracted when re-examined, that a purpose of the LLP's payment was to secure tax relief for its members, something confirmed Mr Afzal submits by the uncommercial nature of the arrangements is sufficient, because of there being at least a duel purpose, to deny the LLP a deduction (see Mallalieu v Drummond at 870).
211. As noted above, some of Mr Timol's answers were the result of the way in which he was questioned. While this may have been true of his answer in relation to the LLP's purpose of securing tax relief, this was not the case with his comment, referred to above, that "the whole thing" was incentivised by the tax structure which, in our judgment, is clearly evidence of the LLP's dual purpose in making the payments. It therefore follows that such payments cannot have been made wholly and exclusively for the LLP's trade, if it had been trading.
R&D Relief
212. Under s 1044 CTA 2009 a company is entitled to corporation tax relief if it meets the conditions A, C and D.
213. Condition A is that the company is a small or medium-sized enterprise in the period (see s 1044(2) CTA 2009). Condition C is that the company carries on a trade (see s 1044(4) CTA 2009) which, for present purposes, despite our conclusion to the contrary we have taken as being met. Condition D is that the company has qualifying Chapter 2 expenditure which is allowable as a deduction in calculating for corporation tax purposes the profits of the trade for the period (see s 1044(5) CTA 2009).
214. A company's "qualifying Chapter 2 expenditure" is defined in s 1051 CTA 2009. This provided:
1051 Qualifying Chapter 2 expenditure
For the purposes of this Part a company's "qualifying Chapter 2 expenditure" means—
(a) its qualifying expenditure on in-house direct research and development (see section 1052), and
(b) its qualifying expenditure on contracted out research and development (see section 1053).
215. Section 1053 CTA 2009 provided, at all material times:
(1) A company's "qualifying expenditure on contracted out research and development" means expenditure—
(a) which is incurred by it in making the qualifying element of a sub-contractor payment (see sections 1134 to 1136), and
(b) in relation to which each of conditions A, C and D is met.
(2) Condition A is that the expenditure is attributable to relevant research and development undertaken on behalf of the company.
(3) [repealed]
(4) Condition C is that the expenditure is not incurred by the company in carrying on activities which are contracted out to the company by any person.
(5) Condition D is that the expenditure is not subsidised (see section 1138).
(6) See sections 1124, 1126 to 1126B and 1132 for provision about when particular kinds of expenditure are attributable to relevant research and development.
216. We find as that £2.9 million (being the sums paid completion of stage 1 and stage 2A) was attributable to R&D condition A is met. Condition C is also met by the LLP and could not be met by NPL which is responsible for undertaking the R&D as a subcontractor.
217. With regards to condition D, s 1138(1)(c) CTA 2009 provided that a company's expenditure is treated as subsidised "to the extent that it is otherwise met directly or indirectly by a person other than the company."
218. This raises an issue as to whether, as Mr Afzal contends, the expenditure is met indirectly by a person other than the LLP because there is borrowing behind it. However, applying the decisions in Quinn (London) Ltd v HMRC [2022] SFTD 152, HMRC v Perenco UK Ltd [2023] STC 1421 and Collins Construction Ltd v HMRC [2024] UKFTT 951 (TC) we agree with Mr Mullan that a loan is not within the scope of what is intended by condition D.
219. The remaining issue in regard to R&D is whether the LLP "incurred" the expenditure on R&D. It is clear from Vaccine Research at [68] that:
"... the question of what, if any, monies were expended 'on' research and development is a 'factual enquiry' which is answered by examining 'the circumstances of each case' (Tower MCashback [2011] STC 1143). ... The 'factual enquiry' must be conducted by reference to a realistic appraisal of the facts: BMBF [2005] STC 1, [2005] 1 AC 684 (at [36]–[38]); Tower MCashback (at [93]) per Lord Hope ... Expenditure which 'produces no economic activity', but rather which goes 'into a loop as part of a tax avoidance scheme', is not expenditure 'on' the acquisition of software rights (Tower MCashback, paras [77]–[78] per Lord Walker)."
220. It is therefore necessary to consider whether the expenditure produced economic activity. Given the completion of stages 1 and 2A of the Task Orders we find that there has been economic activity in relation to the £2.9 million and to that extent, had we found the LLP to have been trading it would have succeeded in relation to the R&D Issue.
True Agreement of the Parties
221. Mr Winter, for HMRC, submits that the Framework Agreement as part of pre-conceived and pre-ordained arrangements, does not comprise the "true agreement" between the parties (ie the LLP and NPL-Sub). The true agreement, he says, was that NPL-Sub would not under any circumstances undertake any R&D itself but would, of the £8 million cash it received from the LLP, deploy a little over £1m on subcontracting the R&D (to NPL). Accordingly, relying on Autoclenz v Belcher [2011] ICR 1157 and HMRC v Atholl House Productions Ltd [2022] 4 All ER 461, Mr Winter contends that it is necessary to apply the particular statutory provisions, eg in relation to wholly and exclusively etc, to that true agreement between that parties to the extent that this differs from the contract between them.
222. However, as Sir David Richards (with whom Arnold and Peter Jackson LJJ agreed) observed in Atholl House, at [147]:
"Lord Clarke, giving the only judgment [in Autoclenz], with which the other Justices agreed, made clear that the well-established principles of interpretation of contracts continued to apply to ordinary contracts, particularly commercial contracts, but there was 'a body of case law in the context of employment contracts in which a different approach has been taken'."
223. Sir David Richards continued, at [153] - [154], by referring to the decision of the Supreme Court in Uber BV v Aslam [2021] UKSC 5, in which Lord Leggatt, who had given the only judgment, had underlined the approach adopted in Autoclenz did not form part of the ordinary principles of contractual construction. It was clear, he said, that in the context of the issue in Autoclenz, the Supreme Court had adopted a different approach but had not fully set out the theoretical justification for this approach.
224. However, in Uber at [69] Lord Leggatt, supplying the theoretical justification, said that it was, in Sir David Richards' words:
"... critical that the relevant rights were not contractual rights but were created by legislation. The task for the tribunals was not to determine whether under the contracts Autoclenz had agreed to pay the valeters the minimum wage but to determine whether they 'fell within the definition of "worker" in the relevant statutory provisions so as to qualify for these rights irrespective of what had been contractually agreed. In short, the primary question was one of statutory interpretation, not contractual interpretation' ([69])."
225. As in Atholl House, the present case does not raise any issue of statutory construction in of a term such as "worker" and, as such we see no justification, as analysed and identified in Uber, for the application of the Autoclenz approach (see at [156] of Atholl House) and for that reason reject Mr Winter's argument on this issue.
Decision
226. Therefore for the reasons above, the appeal is dismissed.
Right to apply for permission to appeal
227. This document contains full findings of fact and reasons for the decision. Any party dissatisfied with this decision has a right to apply for permission to appeal against it pursuant to Rule 39 of the Tribunal Procedure (First-tier Tribunal) (Tax Chamber) Rules 2009. The application must be received by this Tribunal not later than 56 days after this decision is sent to that party. The parties are referred to "Guidance to accompany a Decision from the First-tier Tribunal (Tax Chamber)" which accompanies and forms part of this decision notice.
Release date: 28th FEBRUARY 2025

